Synthesis and Characterization of New Polymers Derivatives from Copoly (Vinyl Chloride - Vinyl Alcohol)
Suror Abdulrahman Mahdi
Department of chemistry, college of science, university of Baghdad, Jadiriya, Baghdad, Iraq.
Corresponding Author E-mail: surchem82@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330523
In this work, new copolymer derivatives linked to poly (vinyl chloride- vinyl alcohol) (1-4) was synthesized via multisteps synthesis. Reactionofpoly (vinylalcohol) (1)with adipoylchloride in presence of benzenegave copoly (vinyloxyadipoy l chladipoyloride) (2) This is the first step. The second step in cluded the reaction of the prepared copolymers with ethanol to give copolymers (vinyloxyadipoy l ester) (3) which containing pendant esters group on polymeric chain. In the fourth step Oxindole subjected to Nucleophilic substitution reaction by vinyloxyadipoyl ester to give N-copolymer Oxindole (4) via Eschweiler-Clarke reaction.The prepared copolymers were identified by FT-IR and 1H-NMR spectra by studying the physical properties such as softening or melting points andsolubility.
KEYWORDS:Polymers; Poly ( Vinyl Alcohol); Poly (Adipoyl Chloride); Oxindole
Download this article as:| Copy the following to cite this article: Mahdi S. A. Synthesis and Characterization of New Polymers Derivatives from Copoly (Vinyl Chloride - Vinyl Alcohol). Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Mahdi S. A. Synthesis and Characterization of New Polymers Derivatives from Copoly (Vinyl Chloride - Vinyl Alcohol). Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=38030 |
Introduction
During the last three decades the role of polymers in biomedicine has seen significant growth. The unique physico-chemical properties offered by polymeric materials have been exploited in a variety of biomedical applications. However, an increasingly important aspect of the field of biomedical polymers is the recognition of the role of polymers as new and novel chemical entities for therapeutic application. For this purpose, the polymer may be intrinsically bioactive, or can be utilized as a carrier for site specific and sustained delivery of chemo- and biotherapeutic agents [1]. On the other hand PVA is an artificial polymer that has been used during the first half of the 20th century worldwide. It has been applied in the industrial, commercial, medical, and food sectors and has been used to produce many end products, such as lacquers, resins, surgical threads, and food packaging materials that are often in contact with food[2] . Promoted by the above facts we report a facile synthesis of new polymer that may be valuable in design and biologically active ,starting from polymer (vinyl alcohol) to
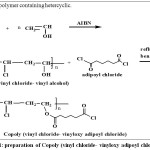 |
Scheme 1: preparation of Copoly (vinyl chloride- vinyloxy adipoyl chloride) Click here to View scheme |
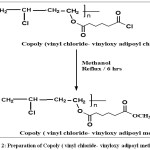 |
Scheme 2: Preparation of Copoly ( vinyl chloride- vinyloxy adipoyl methylate) Click here to View scheme |
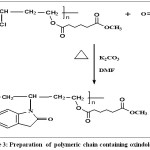 |
Scheme 3: Preparation of polymeric chain containing oxindole. Click here to View figure |
Expermintal
Melting point were determined on Gallenkamp Melting points apparatus(MFB-600),softening points were determined using Reichert thermovar,SP,10\0.25,160. Structures conformation of new prepared copolymer,were provedby FT-IR spectroscopy and other physical properties in cludings of teningpoints, melting points, solubilityof copolymers were measured. All physical properties are usted in Table (1) , Fig (2) H1-NMR and Fig (3) -C13-NMR.
 |
Figure 1: Relationship curve between plasticizer and softening point Click here to View figure |
 |
Figure 2: H1-NMR of compound (3) Click here to View figure |
 |
Figure 3: C13-NMR of compound (3) Click here to View figure |
Preparation of poly (vinyloxy adipoyl chloride)
Mixture 0.01 moleofpoly (vinyl alcohol) and o.o1 mole of adipoyl chloride in benzene used as a solvent were refluxed for 6 hoursat 600 C to givene westerpoly (vinyl oxy adipoylchloride) forming black precipitate [3,4,5], purified by using THF. Conversition of yield 89%.Softening point 189-2010C and melting point 203-2050C
All physical properties are showed in Table(1)
Table 1: Physical properties for new poly (vinyl alcohol) and new ester derivatives
| Com.No | Polymer | time | Yield% | Colour | Melting pointoC | Softening point oC |
| 1 | poly(vinyl alcohol) | 6 hrs. | 78 | Pink. | 186-188 | 161-171 |
| 2 | poly (vinyloxy adipoyl chloride) | 6 hrs. | 89 | Black | 203-205 | 189-201 |
| 3 | poly (vinyloxy adipoyl ester) | 6 hrs. | 84 | gray | 185-189 | 175-190 |
| 4 | Poly (ester oxindole) | 4 hrs. | 78 | Light brown | 168-171 | 153-170 |
Preparation of poly (vinyloxy adipoyl methylate)
Mixture 0.01 moleofpoly (vinyl oxy adipoylchloride) with o.o1 mole of ethanol were refluxed for 6 hoursat 620 C to give newester (grayprecipitate) [6,7,8],which was purified by THF. Conversition of yield 84%.Softening point 175-1900C and melting point 185-1890C physical properties of new polymer are showed in table(1), FT-IR shown in Table (3).
Table 2: Solubility of new polymer
|
NO. |
Benzene |
DMF |
DMSO |
THF |
Water |
CCl4 |
Acetone |
EtOH |
|
1 |
V.S |
V.S |
V.S |
V.S |
P.S |
P.S |
V.S |
V.S |
|
2 |
V.S |
V.S |
V.S |
V.S |
P.S |
P.S |
V.S |
V.S |
|
3 |
V.S |
V.S |
V.S |
V.S |
P.S |
P.S |
V.S |
V.S |
|
4 |
V.S |
V.S |
V.S |
V.S |
P.S |
P.S |
V.S |
V.S |
Preparation of poly (vinyl oxy adipoyl methylate) bearing Oxindole
Oxindole (0.01) mole was treated with (0.01)mole of poly (vinyl oxy adipoylmethylate)in the presense of potassium carbonate (0.5)g and Dimethylformamide as solvent, the mixture was refluxed for 4 hours at 90 0C then cooled, filtered and was hed by hot water to yield light brown precipitate, Conversition of yield 78%.Softening point 153-1700C and melting point 168-1710C physical properties of new polymer shown in table(1),FT-IR shown in Table(3).
Table 3: FT–IR absorption spectra data (cm)-1of new Polymer.
|
Comp. No. |
ν(C-OH) |
ν(C-O) |
ν(C-Cl) |
ν(C-H) aliphatic |
ν(C=O) |
|
1 |
3250-3260 |
1250 |
– |
2990 |
– |
|
2 |
– |
1280 |
617 |
2916 |
1697 |
|
3 |
– |
1242 |
694 |
2923 |
1735 |
|
4 |
– |
– |
– |
2914 |
1668 |
Preparation of Plasticizer
Mixture of solidIgm of PVC [9] with different weight of newester bearing oxindolecopolymer [4,5,6] (vinyl oxy adipoyl methylate oxindole) 0.1gm,0.2 gm,0.3gm,0.4gm,0.5 gm, o.6gm, 0.7gm, 0.8gm, 0.9gm, 1gm of ester with 1gm of PVC give new physical properties Softening point of PVC with copoly (vinyl oxy adipoylmethylate as shownin table (4) and relationship between weight of plasticizer in PVC [10,11,12,13]with softening point shown in curve in fig (1).
Table 4: Relationshipbetweenweightof plasticizer in PVC and softening point.
| plasticizer | Weight% | Softening point C |
| poly (vinyl oxy adipoylmethylate oxindole).which is used with pvc | 0.1 gm+1gm pvc | (187-215) |
| 0.2gm+1gm pvc | (113-138) | |
| 0.3gm+1gm pvc | (128-172) | |
| 0.4 gm+1gm pvc | (123-153) | |
| 0.5 gm+1gm pvc | (70-81) | |
| 0.6 gm+1gm pvc | (115-205) | |
| 0.7 gm+1gm pvc | (71-92) | |
| 0.8 gm+1gm pvc | (61-81) | |
| 0. 9 gm+1gm pvc | (60-79) | |
| 1 gm+1gm pvc | (160-162) |
Result and Discussion
One of the suitable procedure for preparation of poly (vinylalcohol) from (vinylacetate )(1) by hydrolysis in acidic medium with acetone underrefluxe. All physical properties listed in Table(1).The FT-IR spectrum show absorption band at (3250-3600) cm-1 for OH group and at 680cm-1 for C-Cl and 1250 cm-1 for C-O alcohol The FT-IR spectra for poly (vinyloxy adipoyl chloride) (2)showed absorption band at 617 cm-1 for C-Clgroup, and at 1280cm-1 for O-C-Oestergroup, and at 1697 cm-1 for C=O estergroup, and at 2916 cm-1 for C-H group.
Table 5: 1H-NMR spectra for selected copolymers
|
Com.No. |
H1-NMR δ(ppm) |
|
1 |
3.2 (t, 2H, -CH2);2.5(m, 1H, -CH) |
|
3 |
7.89(s,1H,-NH);6.9(s,2H,NH2);3.4(m,2H,-CH2);3.1(t,2H,-CH2);2.8(m,1H,-CH) |
|
4 |
2.78 (s, 3H, CH3); 3.44 (s,2H, -CH2) ; 7.26(t, 2H,-CH2) |
Mechansim of Reaction is Shown in Scheme
The FT-IR spectra fornew poly(vinyl oxy adipoyl methylate ester) showabsorption band[14]at 1735cm-1C=O for ester group, and at 694 cm-1for C-Cl group, and at 2923cm-1for C-H aliphatic group , and at 1242 cm-1 for O-C-O ester group. The FT-IR absorption spectra data (cm)-1of new polymers wasshown in table (3):
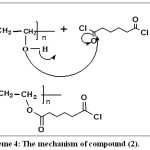 |
Scheme 4: The mechanism of compound (2). Click here to View scheme |
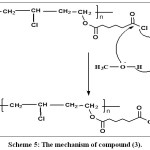 |
Scheme 5: The mechanism of compound (3). Click here to View scheme |
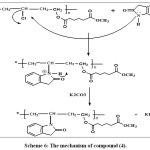 |
Scheme 6: The mechanism of compound (4). Click here to View scheme |
Reference
- Jagur-Grodzinski. J., Erratum to”Biomedical application of functional polymers” ,React. Funct. Polym. 1999.,39 , 99-138.
CrossRef - C.C. Demerlisand D.R. Schoneker, “Review of the oral toxicity of polyvinyl alcohol (PVA)”,Food Chem. Toxicol.,41, 319–326 (2003).
CrossRef - L.E. Coleman and Dunn, “J.Org.Chem.”,24,135. 1959.
CrossRef - Rodriguez,F.”Principle of polymer system”2nd. ed. Mc Graw-Hill Book company,(1983).
- F.W. Billmeyer, “Text book of polymer science”Wiley inter science, New York.(1970).
- T.M.Pyriadi, “Practical polymer chemistry”, University of Mousel press,(1985),Iraq.
- W.R. Roderick and Bhatia,P.L.,J.Org.chem.,28.8 (1963).
- C.E.Rehbery and C.H.Fisher,Polymer,Ind.Eng.chem.40, 1429 (1948).
- J.R.Darbyand J.K. Sears, “Plasticizers”inencyclopediaofpolymerscience andTechnology.10,.228 (1969).
- A.W.Campbell andP.F.Tryon, Ind.Eng.chem.54,125 (1953).
- R.F.Gould, “plasticizationandplasticizerprocesses,Advancein chemistry series NO.48”,American chemical socity,Washington,D.C. (1965).
- G.S.Misra, “Introductionpolymerchemistry”EX-Director,Indianloc Research Institute Namkum,Ranchi,(1993).
- A.K.Doolittle,”The Technology of solvents and plasticizer”, New york, John wiley and sons,inc. p.870 (1954).
- D.H. williams and I.Fleming, “Spectroscopic method inorganic chemistry” Jon wiely and Sons New York, (1958).

This work is licensed under a Creative Commons Attribution 4.0 International License.









