X-Doped Graphene Interaction with Anodic Materiallibs
Hamidreza Jalilian and Majid Monajjemi
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: m_monajjemi@srbiau.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/330505
Thestructures of graphite carbonadoes and hexagonal boron nitride or h-BN, parent materials for carbonadoes and boron nitride nano-compounds are quite similar. The measured reversible Li+ capacities of X-G// (h-BN)// X-G (X=Be, B, N) in the anode materials are extremely improved compared to the graphite structures in the based anodes. In this study Boron nitride sheet has been localized inside two X-graphene electrodes as an option to enhance electrochemical ratio. Additionally, we have found the structure of G-X/(h-BN)n/X-G can be for improving the capacities and electrical transports in the C-BN sheets of LIBs. In addition, the BN sheet modified and designed of X-G/ (h-BN)/X-G structures provide a strategy to improve the yields of BN-G-sheet of anodes. X-G/h-BN/X-G could also be assembled into free standing electrode of any binder (current collector), which will causes for increasing specific energies and densities for the batteries design.
KEYWORDS:Graphene; Doping; Anode Lithium; Ion Battery; LIBs
Download this article as:| Copy the following to cite this article: Jalilian H, Monajjemi M. X-Doped Graphene Interaction with Anodic Materiallibs. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Jalilian H, Monajjemi M. X-Doped Graphene Interaction with Anodic Materiallibs. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=37945 |
Introduction
Thecarbonadoessuch as graphite are layered materials composed of hexagonal lattices sheets; graphite1,2 is including ofcarbon atoms at all direction in the lattice points, while the h-BN is consist of alternating atoms from boron and nitrogen respectively. The lattice constants for graphite and h-BN are 2.46 Å and 2.50 Å repectively1,2.In other side, layered BN is transparent and is an insulator2,3 compare to graphite.The layers of h-BN are arranged as boron atoms in one layer which are located directly2 on the top of nitrogen2 atoms in a neighboring section and vice versa3.In graphite structures, stacking3 is slightly different2whilehexagons are offset2 and cannot lie on the top of each other so interlayer distances can be consider similar: 3.35 Å for graphite1and 3.33 Å for h-BN respectively Fig.1.
Recently, the theoretical approaches have been presented the band structures3 of alone layer of graphite and h-BN whilefor a layer of graphite which called graphene, 2 bands cross each other at the Fermi3 energies. For this matter, graphene is a semimetal4. In contrast graphene, for a alayer of the h-BN, equivalent4 bands cannot cross each other and amount 4.5 eV band gap forms. Via an experiment, h-BN has been calculated to have a band gap around 5.8 eV4.
Crystallographic of BN is classified into four polymorphic3 forms: Hexagonal4 BN (h-BN) (fig 1(a); rhombohedral3,4 BN (r–BN); cubic B-N (c–BN); and wurtzite4 BN (w–BN).
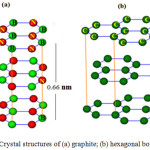 |
Figure 1: Crystal structures of (a) graphite; (b) hexagonal boron nitride Click here to View figure |
In 1842, Balmain5sensitized- BN as reaction products via boric-oxide and potassium cyanide5through1 atmosphere pressure. Then, various methods havebeen reported through h-BN and r-BN which formed under ambient5 pressure and c-BN has been synthesized from h-BN via high pressure and high temperatures. It is notable that w-BN is prepared from h-BN via high pressure at room temperature6.
The discovery of amazing reversible, low-voltage7 Li-intercalation carbonaceous8 materials, Sony Company realized the commercialization7 of xC6/Li 1-x CoO2 cells in 19917. Lithium ion batteries (LIBs) are widely used in small gridstorage systems.
LIBs generally consist of positive and negative electrodes including a conducting electrolytewhere store electrical energy in the two electrodes8 in the form of Li-intercalation8 compounds. Electrodes, separator, and electrolyte are the main components of the LIB where the anode plays an essential role in the performance of these devices.
In the charging mechanism of the LIBs, Li+ released from the negative electrode and move inside the electrolyte then inserted into the positive electrode. In the discharging mechanism,Li+ are extracted8 from the (+) electrode and move back to the (-) electrode. Although the electrolyte establishes high ionic conductivity9 between 2 electrodes, the electrolytes9 are not responsible8,9 for the conduction of free-electrons. So the electrons complete the 1/2 reaction will move in the way of an extra9 external wire.
Various experiments have accomplished for confirming the utilization of graphene in the forms of nano-sheets or nano-ribbons for enhancing lithium storage9 capacities and for improving recharge performance9-11. Furthermore in this study, semi-empirical MM calculations have been donefor investigatingLi+ storage between 2 graphene11sheets12, as well as a few heteroatom-substituted11carbon materials13.
Dis-charging or charging of Li+ in carbonadoes iswell established and arrangedup to now18-21. It has also been exhibitedthe mechanism of the repulsive forces13 in the mixed stages which can result in the pure stages during intercalation19-23. In this study, chargingand discharging of Li-ions has investigated in h-BN with the positive electrode reaction as: Li
![]()
and the negative electrode reaction as:
![]()
while the whole reaction is:
![]()
It has been suggested22 that lithium atoms are stored23 via two mechanisms: intercalation and alloying24.
Recently many works has been established for describing the intercalation and diffusion of Li+ at different sites on carbonadoes graphite and many studies have been performed in order to explain the mechanism by which Li+ s are stored in electrodes, including theoretical works13-24 .
The electricalconductivitiesfor the Li+-Graphene increases25via increasing intercalation levels due to the donor26 nature of electronsfor the lithium. This is in contrast of ionic conduction26 in which diffusivity27 decreases26 due to theinsertion of Li-ions27. In the case of amorphous26 carbon as thedisorder28 increases electrical conductivity28 significantly decreases29.
Recently, Fisher group fabricated29-30 a tube-in-tube structure with Li+ intercalation capacities2 times higher than that of the template-synthesized30-40 Carbon-NTs, as the inner tubules provided more electrochemical40-43 active sites for intercalation of Li ions28-51.
Boron nitride tubes(BN-NT), which firstly predicted and synthesized by Rubio and Chopra respectively40-48, has a structural similar to Carbon-NTs but,in contrast to the Carbon-NTs being metallic40(semiconductor) depending on chirality42-46. BNNTs are usually can be an insulator regardless of their helicity, tube diameters and number of tube walls42-49.
Experimental results have exhibited that small SW-Carbon-NTs are usually found inside multi-walled Carbon-NTs 40-51. There is, therefore49, also a strong motivation to study in detail the stability and interaction of small Boron-Nitride-NTs inside a larger one (viewpoint of diameters), which makes easier to understandthe experimental results. Besides, the study on double-walled Boron-Nitride-NTs (DBN-NTs) has demonstrated an interesting variation in their electronic50 properties when compared with those of freestanding49 component of BNNTs48-51. So it is also important to see the inter-wall coupling behavior and interaction energies associated with the small Boron-Nitride-NTs.
In summary, Graphene and h-BN compounds as one kind of classical materials42 with a mature studied history will play asignificant role in the battery market of the near future,but how to combine hybrid materials together to obtainsafe, stable, and high-capacity electrodes has always beenthe radical problem that many researchers are trying tosolve.
Theoretical Background
Diffus ion properties45-51 of Li-ion cell determines some of the keys performance metrics of Li+ ion batteries cells, consist fo the charges anddischarges rate, practical capacities and cycling stabilities. The governing equation describing the diffusion process is known as Fick’slaw as:
![]()
and
![]()
where “ji” is ionic flux, molcm−2 s−1, Di is diffusivity of solute (i =1, 2), cm2 s−1 and Ci is concentration of species i , (molcm3).The proportionality 40-50 factor D is the diffusivity or diffusion coefficient as
![]()
Li+-ion cells consist of, all Keyes phenomena involves conducting charged particles as a primary cell from cathodes to anode or vice versa as a secondarycell from anode to cathodes.
Typical commercially used lithium-ion battery consists of several interconnected electrochemical cells, where each cell is composed of a graphite anode (such as Meso-carbon microbeads), a cathode formed by lithium metal oxide (such as LiCoO2) and electrolyte (such as LiPF6 dissolved in ethylene carbonate/dimethyl carbonate mixture) embedded in a separator felt.
Anode Materials
In the case of anode, Li metal is found to be the most electropositive (-3.04 V versus standard hydrogen electrode) with large reversible capacity (≈ 4000 A h kg-1). However, due to safety considerations (explosion hazards as a result of dendrite growth during cycling), metallic Li has been substituted by various carbonaceous materials.
During discharge, Li+ ions are extracted from the layered graphite, they pass55 through the electrolytes and intercalate among the LiCoO2 layers (Fig.2.).
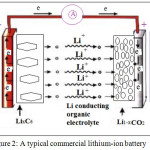 |
Figure 2: A typical commercial lithium-ion battery Click here to View figure |
Density and Energy of Lithium in Diffusion Model
The electron densities has been defined as
![]()
Where ηi is occupational number of orbitals (i), φ are orbitals wave functions, Χ is basis function and C is coefficient matrix, the element of ith row jth column52 corresponds to the expansion coefficient of orbital j respect to basis function i. Atomic unit for electron density can be explicitly 54 written as e/Bohr3
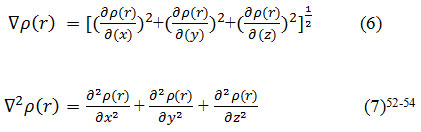
Electron localization and chemical reactivityies55 have been built by Bader55.
The Hamiltonian Kinetics Energies Densities K(r)
The kinetic energies densities are not defined, since it has been expected value for kinetic energies operators
has been recovered via integrating kinetic energies densities from an alternative definition. One of general used definition can be demonstrated as:
![]()
“G(r)” is famous as positive definite kinetic energies densities.
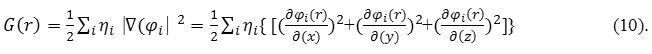
K(r) and G(r) are directly related by Laplacian of electron density
![]()
Electron Localization Function (ELF)
Becke and Edgecombe noted that spherically averaged likespin conditional pair probability has direct correlation with the Fermi hole and then suggested electron localization function (ELF)53-54.
![]()
where
![]()
and
![]()
for close-shell system, since
![]()
D and D0 terms can be simplified as
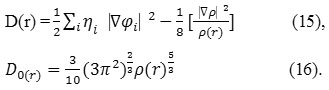
Savin, has reinterpreted the ELF52 in the view of kinetics energies52-58.
![]()
Where
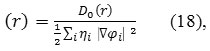
Do (r) for spin-polarized system and close-shell system are defined in the same way as in ELF53.
Local Entropies
Local information entropiesare quantification of information, which was proposed by Shannonto decompose di-atomic and tri-atomic molecules into space by a minimized information entropy52-57.
Parr has discussed the relationship between information entropies and atom partition as well as molecular54 similarity54. Noorizadeh and Shakerzadeh suggested using information entropy to study aromaticity. probability function is
![]()
If P (x) is replaced by
![]()
then the integrand can be called LIE of electrons.
![]()
Where, N is the total number of electrons in current system.
At each inter-tube configuration, a single-point calculation is carried out and the total energy is recorded.The resulting sliding-rotation energy surfaces are used to fix our model in better position.
We further calculated the interaction energy between x lithiuman h-BN sheets.
The interaction energy was calculated via an Mp6 method in all items according to
![]()
Where the “ΔEs ” is the stability energy of system.
The contour line map has drawn via Multiwfn software52-54. The graphs are exhabited on interactive interfaces and the methods in this work has based on our previous work59-99.
Result and Discussion
We have listed the data of Density, energy, electron localization function (ELF), localized orbital locator (LOL) and local entropy, gap energy, charge from ESP, electrostatic potential ,ionization energy,the charges of two doped grap hene electrodes and the stability energy of X-G-(h-BN) -X-G and dielectric in 4 tables (tables1-6) and these data have plotted in 10 the figures (figs.1-10)
We have calculated the gradient norm and the Laplacian of electron density via equals 7 and 8 for the lithium diffused in the X-G/(h-BN)/X-G system respectively and the data are listed in table 1. For calculation the electron spin density from the difference between alpha and beta density, we have Used
![]()
then the spin polarization parameter function will be returned instead of spin density
![]()
The absolute value of ξ going from zero to unity corresponds to the local region going from un-polarized case to completely polarized case Table 1.
In this work it has been calculated the local Information entropies for each of Li atoms via eqs. 19-20 and the integrating of those functions over whole spaces yield the information entropies. The data of local Information entropies are listed in Tables 1-5
Weak interaction (equals 20 and 21) has significant influence on conformation of macro-molecules, however reproduction of electron densities by ab initio and grids data calculation of reduced density gradient (RDG) for such huge systems are always too time-consuming.
In this work it has been exhibited the complex of X-G/(h-BN)/X-G which demonstrate a high electrical conductivity and a good mechanical strength, excellentflexibility, great chemical stabilities and high specific surfaces area. Thoseare especially noticeable when the graphene are chemically converted via greater proportion of functional groups, proving that they are suited for using as the basecomposite electrodes materials as wellas enhancing the electrode’s mechanical stabilities. As a result, X-G/(h-BN)/X-G containing electrode materials have highcapacity and good rate performance. X-G/(h-BN)/X-Gflexibilities makes thoseas an idealmaterial for buffering metal electrode’s volume expansionand contraction during the charge or discharge phenomenon.
Further, the excellent electrical properties of X-G/(h-BN)/X-Gcan enhance the conductivity of metal electrodematerial. Finally, the lithium storage capacity for mostmetal oxide composite materials with X-G/(h-BN)/X-Gcan be useful greatly.
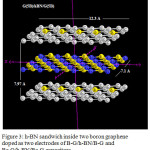 |
Figure 3: h-BN sandwich inside two boron graphene doped as two electrodes of B-G/h-BN/B-G and Be-G/h-BN/Be-G capacitors Click here to View figure |
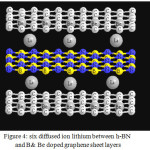 |
Figure 4: six diffused ion lithium between h-BN and B& Be doped graphene sheet layers Click here to View figure |
Table 1: Density, energy, Electron localization function (ELF), Localized orbital locator (LOL) and Local Entropy for each Li of 6 lithium diffused on X-G/(h-BN)/X-G (X=B,N and Be) mixed layers
| LithiumNo. | Density of all electron (10-4) | Density of electron (10-4) | Density of electron (10-4) | Spin density of electron | Potential energy β ensity ( 10-3J) | Hamiltonian kinetic Energy ( 10-4J) | LOL 10-3 | LocalEntropy 10-6 | Ellipticity | ELF 10-7 | Eta index |
| X=B | |||||||||||
| Li+(2) | 0.30 | 0.15 | 0.15 | 0.0 | -0.23 | -0.15 | 0.16 | 0.50 | -0.15 | 0.31 | 0.16 |
| Li+(4) | 0.26 | 0.13 | 0.13 | 0.0 | -0.29 | -0.15 | 0.18 | 0.52 | -0.30 | 0.32 | 0.14 |
| Li+(6) | 0.38 | 0.19 | 0.19 | 0.0 | -0.27 | -0.12 | 0.17 | 0.53 | -0.22 | 0.31 | 0.17 |
| X=Be | |||||||||||
| Li+(2) | 0.22 | 0.11 | 0.11 | 0.0 | -0.28 | -0.14 | 0.21 | 0.50 | -0.19 | 0.38 | 0.17 |
| Li+(4) | 0.26 | 0.13 | 0.13 | 0.0 | -0.27 | -0.13 | 0.18 | 0.54 | -0.12 | 0.35 | 0.13 |
| Li+(6) | 0.34 | 0.17 | 0.17 | 0.0 | -0.25 | -0.15 | 0.19 | 0.51 | -0.41 | 0.36 | 0.16 |
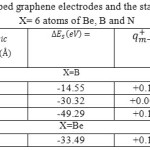 |
Table 2: The Charges of two doped graphene electrodes and the stability energy of x-G/(h-BN)/x-G X= 6 atoms of Be, B and NClick here to View table |
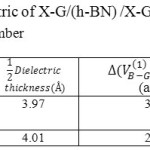 |
Table 3: The dielectric of X-G/(h-BN) /X-G modeled in various Lithium number Click here to View table |
 |
Table 4: Nucleor list and HOMO/LUMO and Gap energy for the B-C/h-BN/B-GClick here to View table |
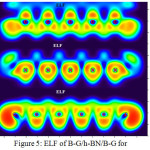 |
Figure 5: ELF of B-G/h-BN/B-G for Click here to View figure |
 |
Figure 6: K(r)of 3 lithium diffusion inside Be-G/h-BN/Be-G |
References
- Tatar,R.C.; Rabii, S, Physical Review B1982, 25, 4126-41.
CrossRef - Sichel,E. K.; Miller, R. E.; Abrahams, M. S.; Buiocchi,C. J. Physical Review B, 1976 13, 4607-11
CrossRef - Hyun,K.Y.; Chang, K. J.; Louie,S. G. Physical Review B, 2001, 63, 205408 4. A. Zunger, Katzir, A.; Halperin,A. Physical Review,1976, B 13 5560-73
- W. H. Balmain, J. Prakt. Chem. 27, 422(1842).
CrossRef - Sato,T.;Report of National Institute for Research in Inorganic Materials, Tsukuba, Japan (1987).
- Yang,Z. G.; Zhang,J. L.; Kintner-Meyer,M .C .W.; Lu,X. C.; Choi,D. W. Lemmon,J. P.; Liu,J.; Chem. Rev.2011,111, 3577–3613
CrossRef - Guerard,D.; Herold,A.; 1975 13:337-345
- Yoo,E. J.; Kim,J.; Hosono,E.; Zhou,H. S.; Kudo ,T.; Nano Lett,2008, 8:2277-2282
CrossRef - Wang,G.; Shen,X. P.; Yao,J.; Park,J.;Carbon, 2009,47:2049-2053 .
CrossRef - Bhardwaj ,T.; Antic,A.; Pavan,B.; Barone,V.; Fahiman,B. D.;JACS,2010, 132:12556-12558
CrossRef - Suzuki,T.; Hasegawa,T.; Mukai,S. R.; Tamon,H.;Carbon,2003, 41:1933-1939.
CrossRef - Hasegawa,T.; Suzuki,T.; Mukai,S. R.; Tamon,H.;Carbon,2004, 42:2195-2200
CrossRef - Noel,M.; Suryanarayanan,V. Journal of Power Sources,2002, 111, 193–209.
CrossRef - Tirado,J.L.;Materials Science and Engineering R , 2003, 40, 103–136.
CrossRef - Fu,L.J.; Liu,H.; Li,C. Wu,Y.P.; Rahm,E.; Holze,R.; Wu,H.Q. Solid State Sciences,2006, 8113–128
- Frackowiak,E.; Béguin,F.;Carbon,2002, 40, 1775–1787
CrossRef - Whittingham,M. S.; Jacobson (Eds.),A.J.;Intercalation Chemistry, Academic Press1982
- Safran,S.A.; Hamann,D.R.;Physical Review Letters.1979, 42 (21), 1410–1413
CrossRef - Levi,M.D.; Aurbach,D.; Maier,J. Journal of Electroanalytical Chemistry, 2008, 624 251–261.
CrossRef - Zabel,H.; Solin (Eds.),S. A.;Graphite Intercalation Compound I, Springer-Verlag, 1990
- Wu,Y.P.; Rahm,E.; Holze,R. Journal of Power Sources,2003,114, 228–236
CrossRef - Lee,J.K.; An,K.W.; Ju,J.B.; Cho,B.W.; Cho,W.I.; Park,D.; Yun,K.S.;Carbon, 2001,39, 1299–1305
CrossRef - C. De las Casas, and W.Z. Li, J. Power Sources,2012,208, 74–85
CrossRef - Z. H. Yang, and H. Q. Wu, Mater. Chem. Phys.2001, 71, 7–11
CrossRef - K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V.Grigorieva, and A.A. Firsov, Science,2004, 306 , 666–669
CrossRef - B. Partoens, and F.M. Peeters, Physical Review B, 2006, 74, 075404-1–075404-11
CrossRef - H. Tachikawa, and A. Shimizu, Journal of Physical Chemistry B,2006,110, 20445–20450
CrossRef - K. Naoi, N. Ogihara, Y. Igarashi, A. Kamakura, . Kusachi, K. Utsugi, Journal of the electrochemicalSociety,2005,152 (6) , A1047–A1053
CrossRef - H. Groult, B. Kaplan, S. Komaba, N. Kumagai, V. Gupta, T. Nakajima, and B. Simon, Journal of the Electrochemical Society,2003,150 (2), G67–G75
CrossRef - M. Endo, Y. Nishimura, T. Takahashi, K. Takeuchi, M.S. Dresselhaus, Journal of Physics and Chemistry of Solids,1996,57 (6–8), 725–728
CrossRef - T. Ohzuku, R.J. Brodd, Journal of Power Sources, 2007,174, 449–456
CrossRef - Monajjemi,M.; Boggs,J.E.;J. Phys. Chem. A,2013,117,1670 −1684
CrossRef - Monajjemi,M.; Lee,V.S. Khaleghian,M.; Honarparvar,B.; Mollaamin,F.;J. Phys. Chem. C. 2010,114 15315
CrossRef - Monajjemi.M.;Journal of Molecular Modeling ,2014, 20, 2507
CrossRef - Monajjemi, M. Theor Chem Acc, 2015, 134:77 DOI 10.1007/s00214-015-1668-9
CrossRef - Monajjemi,M.;Struct. Chem, 2012, 23 551
CrossRef - Monajjemi.M.;Jafari Azan,M.;Mollaamin,F. Fullerenes, Nanotubes, and Carbon Nanostructures. ,2013,21(6), 503–515
CrossRef - Monajjemi,M.;Chemical Physics.2013, 425, 29-45
CrossRef - Novoselov, K.S.; Geim,A.K.; Morozov,S.V.; Jiang,D.; Zhang,Y.; Dubonos,S.V.; Grigorieva,I.V. Firsov,A.A. Science,2004,306 , 666–669
CrossRef - B. Partoens, and F.M. Peeters, Physical Review B,2006,74 075404-1–075404-11
CrossRef - Monajjemi,M.; Mollaamin,F.;Journal of Cluster Science,2012, 23(2), 259-272.
CrossRef - L. Ravagnan, P. Piseri, M. Bruzzi, S. Miglio, G. Bongiorno, A. Baserga, C.S. Casari, A. Li Bassi, C. Lenardi, Y. Yamaguchi, T. Wakabayashi, C.E. Bottani, and P. Milani, Physical Review Letters ,2007, 98, 216103-1–216103-4
CrossRef - Wu,G.T.; et al., Journal of Power Sources,1998 75 175
CrossRef - Jalilian,H.; Monajjemi,M.;Japanese Journal of Applied Physics.2015,54, 085101
CrossRef - Chew,S.Y.; et al., Carbon, 2009,47, 2976.
CrossRef - Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22(4), 673-692
CrossRef - E. Frackowiak, S. Gautier, H. Gaucher, S. Bonnamy, F. Beguin, Carbon,1999,37 (1999) 61.
- Monajjemi, M.; Wayne Jr,Robert.;Boggs,J.E. Chemical. Physics. 2014,433 , 1-11
- S. Y. Chew, S. H. Ng, J .Wang, P. Novak, F. Krumeich,S. L. Chou, J. Chen, and H . K. Liu. Carbon,2009, 47: 2976
CrossRef
- E. Frackowiak, S. Gautier, H. Gaucher, S. Bonnamy, and F. Beguin, Carbon,1999, 37 61
- Joseph, Doninger, Z. Vyacheslav, Barsukov (Eds.), New Carbon Based Materials for Electrochemical Energy Storage Systems, Springer, 2006, pp. 269–276
- T. Lu, F. Chen, Acta Chim. Sinica, 2011, 69, 2393-2406
- T. Lu, F. Chen, J. Mol. Graph. Model,2012, (38): 314-323
CrossRef - T. Lu, F.Chen Multiwfn: A Multifunctional Wave-function Analyzer, J. Comp. Chem. 2012, (33) 580-592
- R.F.W. Bader, atoms in Molecule: A quantum Theory (Oxford Univ. press, Oxford, (1990).
- Becke and Edgecombe J. Chem. Phys., 92, 5397 (1990).
- A. Savin, Angew. Chem. Int. Ed.Engl., 31, 187 (1992).
CrossRef - J.P. Perdew, K.Burke , Ernzerhof, Phys. Rev. Lett. 77, 3865-3868 (1996).
CrossRef - Chegini, H.; Mollaamin, F.; Farahani, P. Fullerenes, Nanotubes, and Carbon Nanostructures. 2011,19, 469–482
CrossRef - Afsharnezhad ,S.; Jaafari , M.R.; Abdolahi ,T.; Nikosade ,A.; Monajemi ,H.; Russian Journal of physical chemistry A, 2007,2,1956-1963
- Baei, M.T.;Mollaamin, F. Russian Journal of Inorganic Chemistry. 2008, 53 (9),1430-1437
CrossRef - Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
CrossRef - Mollaamin, F.; Monajjemi, M, Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
CrossRef - Mollaamin, F.; Varmaghani, Z.; Monajjemi, M, Physics and Chemistry of Liquids. 2011, 49 318
CrossRef - Nafisi, S.;Monajemi, M.;Ebrahimi, S. Journal of Molecular Structure. 2004,705 (3)35-39
CrossRef - Fazaeli, R.; Monajjemi, M.; Ataherian, F.; Zare, K. Journal of Molecular Structure: THEOCHEM.2002, 581 (1), 51-58
CrossRef - Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.; Russian Journal of Physical Chemistry A , 2008 , 82 (13), 2277-2285
- Monajjemi, M.; Seyed Hosseini, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21, 381–393
CrossRef - Monajjemi, M.; Faham, R.; Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures,2012 20, 163–169
CrossRef - Mollaamin, F.; Najafi, F.; Khaleghian, M.; Khalili Hadad, B.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 201119, 653–667
- Mollaamin, F.; Baei, MT.; Monajjemi, M.; Zhiani, R.; Honarparvar, B.; Russian Journal of Physical Chemistry A, Focus on Chemistry, 2008, 82 (13), 2354-2361
CrossRef - Heshmat, M.; Aghaei, H.; Ahmadi, R.; Zare, K. Bulletin of the Chemical Society of Ethiopia, 2007,21 (1)
- Monajjemi, M.;Honarparvar, B. H. ; Haeri, H. ; Heshmat ,M.; Russian Journal of Physical Chemistry C. 2006, 80(1):S40-S44
- Monajjemi, M.; Ketabi, S.; Amiri, A. Russian Journal of Physical Chemistry, 2006, 80 (1), S55-S62
- Yahyaei, H.; Monajjemi, M.; Aghaie, H.; K. Zare, K. Journal of Computational and Theoretical Nanoscience. 2013, 10, 10, 2332–2341
CrossRef - Monajjemi, M.; Mollaamin, F. Journal of Computational and Theoretical Nanoscience, 2012, 9 (12) 2208-2214
CrossRef - Monajjemi, M.; Honarparvar, B.; Nasseri, S. M. .; Khaleghian M. Journal of Structural Chemistry. 2009, 50, 1, 67-77
CrossRef - Monajjemi, M.; Aghaie, H.; Naderi, F. Biochemistry (Moscow).2007,72 (6), 652-657
CrossRef - Ardalan, T.; Ardalan, P.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 687–708
CrossRef - Monajjemi, M.; Najafpour, J.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21(3), 213–232
CrossRef - Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics, 2013, 19, 155–164
CrossRef - Tahan, A.; Monajjemi, M. Acta Biotheor,2011, 59, 291–312
CrossRef - Lee, V.S.; Nimmanpipug, P.; Mollaamin, F.; Kungwan, N.; Thanasanvorakun, S..; Monajjemi, M. Russian Journal of Physical Chemistry A, 2009, 83, 13, 2288–2296
- Monajjemi, M.; Heshmat, M.; Haeri, HH, Biochemistry (Moscow), 2006, 71 (1), S113-S122
CrossRef - Monajjemi, M.; Yamola, H.; Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 595–603
CrossRef - Monajjemi, M.; Ahmadianarog, M. Journal of Computational and Theoretical Nanoscience. 2014, 11(6), 1465-1471
CrossRef - Mollaamin, F.; Monajjemi, M.Physics and Chemistry of Liquids .2012, 50, 5, 2012, 596–604
- Monajjemi, M.; Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
CrossRef - Monajjemi, M.; Baheri, H.; Mollaamin, F. Journal of Structural Chemistry.2011 52(1), 54-59
CrossRef - Mahdavian, L.; Monajjemi, M.; Mangkorntong, N. Fullerenes, Nanotubes and Carbon Nanostructures, 2009, 17 (5), 484-495
CrossRef - Monajjemi, M.; Mollaamin,F.; Gholami, M. R.; Yoozbashizadeh,H.; Sadrnezhaad, S.K.; Passdar, H.;Main Group Metal Chemistry,2003, 26, 6, 349-361
CrossRef - Monajjemi, M.; Farahani, N.; Mollaamin, F. Physics and Chemistry of Liquids, 2012, 50(2) 161–172
CrossRef - Monajjemi, M.; Seyed Hosseini, M. Journal of Computational and Theoretical Nanoscience .2013, 10(10), 2473-2477
CrossRef - Monajjemi, M.; Khaleghian, M.; Mollaamin, F. Molecular Simulation. 2010, 36, 11, 865–
- Monajjemi, M. Biophysical Chemistry. 2015 207,114 –127
CrossRef - Monajjemi, M.; Heshmat, M.; Haeri, H.H. Biochemistry (Moscow).2006, 71, 113-122
CrossRef - Amiri, A.; Babaeie, F.; Monajjemi, M.Physics and Chemistry of Liquids. 2008, 46, 4, 379-389
CrossRef - Monajjemi, M.; Heshmat, M.; Haeri, H.H.; Kaveh, F. Russian Journal of Physical Chemistry A,2006, 80, 7, 1061-1068
- Mahdavian, L.; Monajjemi, M. Microelectronics Journal. 2010,41(2-3), 142-149
CrossRef - Amiri, A.; Monajjemi, M.; Zare, K.; Ketabi, S.Physics and Chemistry of Liquids. 2006, 44, 4, 449-456.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









