Synthesis of flavanones using methane sulphonic acid as a green catalyst and comparision under different conditions
R. B. Kshatriya, G. M. Nazeruddin
Post Graduate Department of Organic Chemistry and Research Centre,Poona College of Arts,Science and Commerce,(Affiliated to University of Pune) Camp,Maharashtra,India
DOI : http://dx.doi.org/10.13005/ojc/300264
Article Received on :
Article Accepted on :
Article Published : 04 Jun 2014
Flavonoids are an important class of natural products with wide range activities.Flavonoids includes flavone,flavanone,flavane & flavanol.The synthetic route invovles synthesis of chalcone followed by ring closing to give flavanone.So many catalysts were mentioned in past literature.But most efficient catalyst is methane sulphonic acid.It is easy to handle,less reaction time &easily available.Flavanones were synthesized from chalcone using methane sulphonic acid under thermal condition,microw wave and ultrasound condition.Flavanones are syntheisized in very less time compared to other conditions.
KEYWORDS:Flavanone; methanesulphonic acid; thermal; micro wave; ultrasound.
Download this article as:| Copy the following to cite this article: Kshatriya R. B, Nazeruddin G. M. Synthesis of flavanones using methane sulphonic acid as a green catalyst and comparision under different conditions. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Kshatriya R. B, Nazeruddin G. M. Synthesis of flavanones using methane sulphonic acid as a green catalyst and comparision under different conditions. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3571 |
Introduction
Natural products includes alkaloids,terpenoids, coumarins,amino acid derivatives and flavonoids. Flavonoids are found in plant as a secondary metabolite acts as defence from bacteria and viruses.They also acts as coluring agent in flowers to attract insect,honeybees and butterfy toward flower.Due to which pollination occurs easily in plants. Flavonoids are also important for human health as it present in fruits,vegetables and flowers.Initially it is named as Vitamin P.But due to its yellow colour it is named as flavonoids.
Flavonoids are mainly classified as flavone, flavanone and flavane etc1.Human interest is to isolate and synthesize flavone skeleton having pharmacological activity.From past literature we found that flavonoid skeleton (C6-C3-C6 & two oxygen atoms) have wide range of activities.Synthetic and naturally occurring flavonoids i.e. flavanone have interesting pharmaceutical activity like anticancer1,anti-estrogen2,antimycobacterial3,antimicrobial4,anti-lungcancer5,anti-bacterial6,anti-viral7,anti-tuberculosis8,antifungal9,anti-oxidant10,anti-arrhythmic11,antihypertensive12,anti-proliferative13 etc.
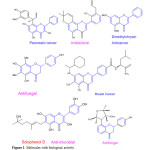 |
Figure 1: Molecules with biological activity Click here to View Figure |
Flavonoids are synthesized by various methods like Clause-Schmidt, Baker-Venkatraman, Ganguly’s method and Robinson’s method etc.13 Armoatic aldehydes and ketones gives chalcone which on cyclization gives flavanone.In past methods chalcone are cyclized to flavanone by I2/DMSO15,AcOH/H2SO416,silicagel17,poly phosporic acid18,TFA19,N-methyl imidazole20,alkali metal carbonates21,KOH/MeOH22,pyridine23,DBU/MW24,HBr/AcOH25,pottasium ferricynide26etc.We synthesized flavanone by using methane sulphonic acid under different conditions.
Methodology
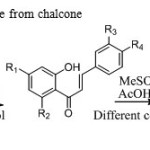 |
Scheme1:Synthesis of flavanone from chalcone Click here to View Scheme |
Experimental
All material purchased from Sigma-Aldrich and solvents from Merck Chemical India.Melting points determined in parafin bath.IR &1HNMR spectra gives structure of compound.
Representative procedure
Thermal condition
A mixture of substituted 2- hydroxy chalcone (1mole) was added in RBF.Then arrange the apparatus with thermometer in oil bath.Place apparatus on digital hot plate.Add through neck of RBF acetic acid and then add dropwise MSA (15 mole%).Maitain temperature 105-1150C till completion of reaction.Reaction was monitored by TLC.Pour the reaction mass in water,filter to get solid.Recrystalise with suitable solvent.Calculate yeild, M.P.After checking solubility in suitable solvent it was given for spectral analysis.
Ultra sound condition
A mixture of substituted 2- hydroxy chalcone (1mole) and methane sulphonic acid was added(15 mole %) in RBF.Add acetic acid minimum to dissolve the reaction mass.Then keep RBF in a water bath with ultrasound.Mantain temp.950C.Carry out reaction for 30 minute under ultrasound.Check TLC.Carry out work up as above.
Under micro-wave condition
A mixture of substituted 2- hydroxy chalcone (1mole) and methane sulphonic acid was added(15 mole %) in RBF.Add acetic acid minimum to dissolve the reaction mass.Then RM subjected to micro-wave.Check TLC.Carry out work up as above.
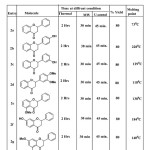 |
Table1:Synthesis of flavanones under thermal microwave and ultrasound conditions Click here to View table |
Spectral Data
2-phenylchroman-4-one (2a)
Colour-light yellow/white powder,M.P.-760C,TLC system-Hex+E.A.(7:3),Soluble – CHCl3
IR (KBr) Vmax/cm-11720,1675,1616-1500,1300,750,690.
1H NMR (CDCl3) d 5.51(d,1H,J = 7 Hz),3.20(d,2H,J=7 Hz),7.40(dd,1H,J = 7.5,1.5 Hz),
7.0(1H,m,J= 7.5,1.5 Hz),7.50(dd,1H,J = 7.5,1.5 Hz),7.15(dd,1H,J = 7.5,1.5 Hz),7.32
(dd,2H,J = 7.5,1.5 Hz),7.25(dd,1H,J= 7.5,1.5 Hz),7.22(m,1H,J= 7.5,1.5 Hz)
2-(4-hydroxyphenyl)chroman-4-one (2b)
Colour-light yellow powder,M.P.-1800C,TLC system-Hex+E.A.(7:3),Soluble – DMSO
IR (KBr) Vmax/cm-1: 3480,1700,1692, 1608, 1514,754,697.
1H NMR (DMSO) d 5.53(d,1H,J=7Hz),3.28(d,2H,J=7 Hz),9.79(s,1H,-OH),6.78(d,2H,
J= 7.5,1.5 Hz),7.33(dd,2H,J = 7.5,1.5 Hz),7.34(d,1H,J = 7.5,1.5 Hz),7.046(m,1H,
J=7.5,7.2,1.5 Hz),7.55(dd,1H,J = 7.5,1.5 Hz),7.76(m,1H,J=7.2,7.2,1.5 Hz),7.76(m,1H
J = 7.6,7.2,1.5 Hz).
2-(3-hydroxyphenyl)chroman-4-one(2c)
Colour-white powder,M.P.-1340C,TLC system-Hex+CHCl3+Acetone.(6:3:1),Soluble– DMSO
IR (KBr) Vmax/cm-13400,1711,1656,1610,1515,750,690.
1H NMR (DMSO) d 5.70(d,1H,J=7Hz),3.17(d,1H,J = 7Hz),6.75(dd,1H,J = 1.5,1.5 Hz),6.92(m,1H,J=
7.5,1.5,1.4 Hz),7.09(m,1H,J =7.6,1.5,1.5),7.21(m,1H,J = 7.5,1.5,1.5 Hz),7.57(dd,1H,J = 7.5,1.5 Hz),7.60
(dd,1H,J = 7.5,1.5 Hz),7.76(dd,1H,J = 7.5,1.5 Hz),7.78(m,1H,J = 7.5,1.5,1.5 Hz)
2-(3,4-dimethoxyphenyl)chroman-4-one (2d)
Colour-light yellow powder,M.P.-1340C,TLC system-Hex+E.A.(7:3),Soluble– DMSO.
IR (KBr) Vmax/cm-11700,1656,1600,1512,740.
1H NMR (DMSO) d 5.42(d,1H,J = 7 Hz),3.14(d,2H,J = 7 Hz),3.92(s,3H,-OMe)
3.90(s,3H,-OMe),6.90(d,1H,J =7 Hz),7.008(d,1H,J=7Hz),7.027(d,1H,J = 1.5 Hz),
7.15(dd,1HJ = 7.5,1.5 Hz),7.52(dd,1H,J = 7.5,1.5 Hz),7.94(m,1H,J= 7.5,1.5,1.5,1.5 Hz)
5-methoxy-2-phenylchroman-4-one (2e)
Colour-Yellow powder,M.P.-1410C,TLC system-Benzene+E.A.(9.5+0.5),Soluble–CHCl3.
IR (KBr) Vmax/cm-11720,1654,1600,1500,1300,980.
1H NMR (CDCl3) d 5.50 (d,1H,J = 7 Hz),3.19(d,2H,J = 7 Hz),2.80(s,3H),
6.72(2H,d,J = 7.5 Hz),7.50(d,1H,J = 7.5 Hz),7.35(s,5 H)
7-hydroxy-2-phenylchroman-4-one (2f)
Colour-Yellow powder,M.P.-1880C,TLC system-Hex+E.A.(7:3),Soluble–CHCl3.
IR (KBr) Vmax/cm-13500,1717,1660,1500,1300,980.
1H NMR (CDCl3) d 5.51(d,1H,J = 7 Hz),3.40(d,2H,J = 7 Hz),6.46(dd,1H,J=7.5,
1.5 Hz),6.50(d,1H,J = 1.5 Hz),7.50(d,1H,J = 7.5 Hz),7.40(dd,2H,J=7.5,1.5 Hz),
7.35(m,3H,J = 7.5,1.5 Hz).
5,7-dimethoxy-2-phenylchroman-4-one (2g)
Colour-/white powder,M.P.-1440C,TLC system-CHCl3+MeOH.(9:1),Soluble – CHCl3
IR (KBr) Vmax/cm-117021674, 1608, 1489,1300,943.
1H NMR (CDCl3) d 5.51(d,1H,J = 7 Hz),3.029(d,2H,J= 7Hz),2.79(s,3H),2.62
(s,3H),6.16(d,1H,J= 1.5 Hz),6.10(d,1H,J = 1.5 Hz),7.38(m,1H,J = 1.5,1.5 Hz),
7.41(m,1H,J = 7.5,1.5,1.5 Hz),7.45(m,1H,J=7.5,1.5,1.5,1.5 Hz)
Results and Discussion
Methane sulphonic acid is used as an acid catalyst in organic reactions because it is a non-volatile, strong acid that is soluble in organic solvents. Methanesulfonic acid is convenient for industrial applications because it is liquid at ambient temperature, while the closely related to para toluene sulphonic acid which solid at room temperature.MSA is considered as intermediate between sulphuric acid and methyl sulphonyl methane.It maintains PH which is necessary to go reaction smoothly.So many acids like acetic acid, sulphuric acid, pTSA, poly H3PO4,Hydrochloric acid were used in previous method.Sometimes mixture of acids,mixed solvents are used in previous methods.So as per our opinion PH is important for the acidic synthesis of flavanone from 2-hydroxy chalcones.We also compared reaction under various conditions like ultrasound,thermal and microwave conditions.
Conclusion
We found cheap,environmental friendly process for the synthesis of flavanones.Methane sulphonic acid is green catalyst compared to other catalysts.We compare different conditions for the reaction.We concluded that reaction under MW will complete in minimum time.
Acknowledgment
We specially thanks to Dr. Rajeshwari Nair,Vice Principal, S.S.R.College of Arts,Commerce & Science, Silvasa (Affiliated to University of Pune) to give lab facility to carry research.
References
- (a)Abou EI-Fotooh G Hammam;Osama I Abd EI-Salam; Ashraf Mohamed ;Nagla Abdel Hafez.Ind.J.Chem.2005,44,1887-1893.(b)Kshatriya,R.B.;Nazeruddin,G.M.OIIRJ,2014 (manuscript accepted)
- Biddle,M.M.;Lin,M.;Sheidt,K.A. J.Am. Chem.Soc.2007,129,3830.
- Soizic, P.; Janin,Y.L.;Brigitte ,Saint-Joanis;Priscille, B.; Sylvie,M.;Koch,M.; Cole,S.T.; François, T.; Pierre-Etienne ,B. Bioorg. Med .Chem. 2007,15,2177- 86.
- Sherif B Abdel Ghani;Louise W.; Zidan H. Z.; Hussein M. Ali;- C.William-Keevil; Brown.,R.C.D.Bioorg. Med. Chem.2008,18,518- 522.
- Ehsan Ullah Mughal; Muhammad Ayaz; Zakir Hussain; Aurangzeb Hasan,- Amina Sadiq; Muhammad Riaz; Abdul Malik; Samreen Hussain; M. Iqbal Choudhary. Bioorg Med Chem 2006,14,4704-471.
- Pandey,V.K.; Singh,V.K.; Tandon,M.; Joshi,M.N.;Bajpai,S.K. Ind. J. Chem.2004,43B,1770-1773.
- Yuh-Meei Lin, Yasheen Zhou, Michael T Flavin, Li-Ming Zhou, WeiguoNie, Fa-Ching Chen. Bioorg Med Chem 2002,10,2795-2802.
- Dandia,A.; Singh,R.; Khaturia,S.Bioorg Med Chem.2006,14, 1303-1308.
- Tapas,A.;Sakarkar,D.; Kakde,R. J.Pharm and Tech. (2008),1(3)132-143.
- Maria Koufaki;Christi Kiziridi;Panagiota Papazafiri;Athanasios Vassilopoulos;Andras Varro;Zsolt Nagy;Attila Farkas;Alexandros Makriyannis.Bioorg Med Chem 2006,14,6666-6678.
- Amit Tapas,Dinesh Sakarkar,Rajendra Kakde, Research J.Pharm and Tech.(2008),1(3),132-143.
- Agarwal,A.D. Int.J.Of Pharmaceutical Science and Nanotechnology 2011,4,1394-1398.
- Kshatriya,R.B.;Nazeruddin,G.M.;Shaikh,Y.I. Oriental J. of Chemistry 2013,29(4),1475.
- Kshatriya,R.B.;Machhi,J.K.;Patil,M.S.;Patel,A.C.;More,D.B. Int.-Multi. e – Journal,2013,II(X),67-79. Kshatriya,R.B.;Machhi,J.K. International Journal of Phrama research and Review (Manuscript Accepted Feb.2014 issue).
- Lokhande,P.D.; Sakate,S.S.; Taksande,K. N.; Navghare,B.Tetrahedron Lett. 2005,46,1573.
- Babber, S.; Chandra, S.; Aggarwal, A. K. Indian J.Chem. 1987, 26B, 797. Sangwan, N. K.; Varma, B. S.; Dhindsa, K. S. Chem. and Ind.1984, 271.
- Paquette, L. A. Encyclopedia of Reagents for Organic Synthesis; John- Wiley &Sons: New York, U. S. A., 1995, 4172.
- Chaturvedi, R.; Patil, P. N.;Mulchandani, N. B. ibid. 1992, 31B, 340.
- Liu,B. K. , Wu, X. ;Qian,Q.; Lv,D.S. Lin, X. F. Synthesis 2007,2653.
- Mondal,R. Gupta, A.D.Mallik, A.K. Tetrahedron Lett. 2011,52, 5020.
- Vatkar,B.S.;Pratapwar,A.S.;Tapas,A.R.;Butle,S.R.Tiwari,B.
- International Journal of ChemTech Research 2010,2, 1, 504-508.
- Dutta, C. P.; Roy, L. P. K. Indian J. Chem. 1975, 13, 425.
- Patonay, T.; Varma, R. S.; Vass, A.; Levai, A.; Dudas, J.Tetrahedron Lett. 2001, 42, 1403.
- Lee,J.I ; Jung,M.G. Bull. Korean Chem. Soc. 2005, 26(12), 2045.
- Makrandi, J. K.; Bala, S.Syn. Comm. 2000, 30, 3555.

This work is licensed under a Creative Commons Attribution 4.0 International License.









