Synthesis and Molecular Docking Studies of Novel 2-(2-Amino-6-Phenyl-4-Pyrimidinylamino)Ethanol Derivatives: Using Ring-Opening Reactions of Cyclic Ketene-N,O-Acetal.
Ravi Kumar G. M. V. N. A. R. and Arumugam Thangamani
Department of Chemistry, Karpagam University, Karpagam academy of higher education, Coimbatore-641021, Tamil Nadu, India.
Corresponding Author E-mail: dr.rav.chem@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330361
A series of six novel 2-(2-amino-6-phenyl-4-pyrimidinylamino) ethanol derivatives have been synthesized starting from commercially available substituted acetophenones via OxoketeneDithioacetals with high yields. Ketene dithioacetals to react with 2-aminoethanol or l-amino-2-propanol to afford the corresponding substituted 2-methyleneoxazolidines which are utilized in the synthesis of 6-aryl-2-amino-4-pyrimidinamine Derivatives. The molecular docking studies revealed that all the synthesized compounds best fit into the active site of HDAC2, anti-cancer protein.
KEYWORDS:2-amino-pyrimidine Derivatives; Ketene dithioacetals; Cyclic Ketene N; O-acetal; Molecular docking
Download this article as:| Copy the following to cite this article: Kumar G. M. V. N. A. R. R, Thangamani A. Synthesis and Molecular Docking Studies of Novel 2-(2-Amino-6-Phenyl-4-Pyrimidinylamino)Ethanol Derivatives: Using Ring-Opening Reactions of Cyclic Ketene-N,O-Acetal. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Kumar G. M. V. N. A. R. R, Thangamani A. Synthesis and Molecular Docking Studies of Novel 2-(2-Amino-6-Phenyl-4-Pyrimidinylamino)Ethanol Derivatives: Using Ring-Opening Reactions of Cyclic Ketene-N,O-Acetal. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33352 |
Introduction
Cancer is a primary cause of death globally, impacting above 14 million people per annum. Among various types of cancers, Colon cancer is the most common type. Throughout the world, this cancer is the most frequently diagnosed (second in women and third in men) and it consistently lies within the five most serious types of cancer each year. This fact clearly emphasizes the need for detection of novel chemotherapeutic agents with potent anticancer activities. The core moiety of several anticancer agents comprised the pyrimidine nucleus which acts as tyrosine kinase inhibitors. Pyrimidine containing compounds are renowned for their biological activities due to the existence of pyrimidine base in genetic material of cells particularly in thymine, cytosine and uracil, which form the building blocks of DNA and RNA. 6-aryl-2,4-Diamino pyrimidine (I) derivatives are known to exhibit important biological activities such as antifilarial topoisomerase II inhibitors1, MTH-1 inhibitors for treating cancer3, anti-nflammators4and they are known to show potential antiplasmodial agents2. But the synthesis and biological activity of 2-(2-amino-6-phenyl-4-pyrimidinylamino)ethanol (I) derivatives are not much investigated. These pyrimidine derivatives were previously synthesized from ketene S,S acetals by treating with guanidine11. And these pyrimidine derivatives having sulfide group was oxidized to to sulphone and substituted with amine1, 2, 6 (scheme-1). But the main disadvantage involves nucleophilic Substitution on pyrmidine ring having sulphone group need conditions.
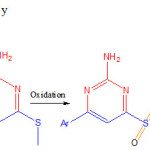 |
Scheme 1: Earlier methodology |
The other drawback is more number of steps are required in this strategy, thereby combined yield seems to be less (scheme 1).
Aim and Objectives
The 2-(2-amino-6-phenyl-4-pyrimidinylamino)ethanol derivatives were planned to synthesize in the aim of exploring their anticancer, antibacterial and antimicrobial activity. Here by, we are proposing a versatile novel method to synthesize these compounds in very high yields (via α-OxoketeneDithioacetals) from commercially available and cheap starting materials such as acetophenones. Here, we describe the synthesis of some new heterocyclic ketene N,O-acetals and their reactions with guanidine hydrochloride.
The other drawback is more number of steps are required in this strategy, thereby combined yield seems to be less (scheme 1).
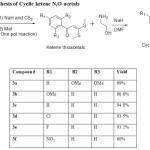 |
Table 1: Synthesis of Cyclic ketene N,O-acetals |
Retrosynthetic Analysis
2-(2-amino-6-aryl-4-pyrimidinamino) ethanol (I) derivatives could be synthesized from guanidine and 2-Oxazolidin-2-ylidene-1-phenylethanone (II), five-membered cyclic ketene-N,O-acetals. These five-membered cyclic ketene-N,O-acetals can be achieved from commercially available substituted acetophenones through ketene-S,S-acetals (Scheme 2).
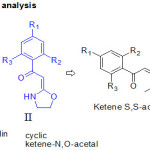 |
Scheme 2: Retrosynthetic analysis |
Results and Discussion
The synthetic strategy started from the commercially available substituted acetophenones. The substituted acetophenones were treated with NaH and CS2 followed by methylation using MeI, resulted the α-Oxoketene dithioacetals in a one pot reaction7-9 (Scheme 1). Further these ketene dithioacetal were treated with 2-ethanolamine under basic conditions yielded the required Cyclic N,O-ketenecetals10 (3a-3f). From the yield values in the tabular result (table-1), it is clearly showing that ketene dithioacetals having electron donating groups on aromatic ring are much favorable for the reaction. Whereas ketene dithioacetals having electron withdrawing groups on aromatic ring has comparably less reactivity, because of the delocalization of the electron cloud and stabilsation of the molecule. 1H- and 13C-NMR spectra of the synthesized cyclic N,O-ketenecetals(3a-3e) shows the presence of only one set of signals, representing that these compounds are not a mixture of E and Z isomers. Because of the presence of intra molecular hydrogen bond (as shown in figure 1) these
 |
Scheme 3 |
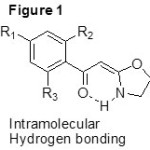 |
Figure 1 Click here to View figure |
These Cyclic N,O-ketene acetals were made to react with guanidine hydrochloride using
potassium tert. Butoxide as base resulted the required 2-(2-amino-6-aryl-4-pyrimidinamino) ethanol Derivatives (4a-4f). From the yield values in tabular result (table-2), it is showing that electron withdrawing groups on aromatic ring is favorable for the reaction. The structures of all the six compounds were fully characterized by 1H NMR, 13C NMR, Mass and IR.
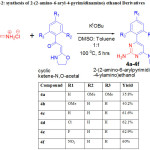 |
Table 2: Synthesis of 2-(2-amino-6-aryl-4-pyrimidinamino) ethanol Derivatives |
Plausible Mechanism
A postulated reaction pathway for the formation of product undergoes Michael addition with push–pull alkene, which was followed by intramolecular cyclization by the breakage of C- bond to afford required products in good yields.
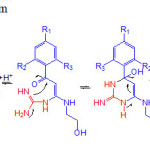 |
Figure 2: Plausible mechanism |
Docking Studies
Materials and Methods
Ligand Preparation
Geometry optimized 2D structure of synthesized molecules was sketched and prepared for docking by using Ligprep, a versatile program of Schrödinger suite 2015 and minimized using OPLS-2005 force field. A total 10 conformation were generated.
Protein Preparation
Crystal co-ordinates for HDAC2 (PDB ID: 3MAX) were taken from protein databank (http://www.rcsb.org). Protein was prepared for docking studies using protein prep wizard of Schrödinger 2016 (Maestro 11, Schrödinger, LLC, New York, NY). Bond orders and formal charges were added for hetero groups, and hydrogen’s were added to all atoms in the system. Water molecules with in 5A0 distance were removed. For each structure, a brief relaxation was performed using an all-atom constrained minimization carried out with the Impact Refinement module using the OPLS-2005 force field to alleviate steric clashes that may exist in the original PDB structure. The minimization was terminated when the energy converged or the RMSD reached a maximum cut off of 0.30 Å.
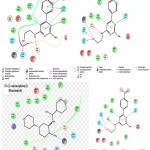 |
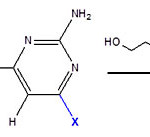
Figure 3: Ligand diagrams |
Grid Generation
Glide energy grid was generated using binding pocket of N-(2-aminophenyl) Benzamide with Grid co-ordinates of X: 66.51, Y: 29.55, Z: 1.38 in anticancer HDAC2 protein.
Glide Docking
All the synthesized compounds (4a-4f) were docked into active site pockets of corresponding protein by using GLIDE13 module of Schrödinger. Standard precision docking was performed. A total of 10 ligand conformations were allowed and finally top score conformation was selected as active conformation. Molecules were analysed based on docking score, interacting amino acids, and hydrogen bonds (Table 3). N-(2-aminophenyl) Benzamide was considered as standards for HDAC2 respectively.
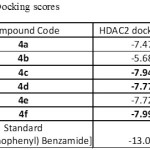 |
Table 3: Docking scores Click here to View table |
 |
Scheme 4 Click here to View scheme |
Experimental Section
General procedure for the synthesis of Cyclic N,O-kenteneacetal
To the starting material (ketene diothio acetal) taken in DMF, 2 equivalents of ethanolamine was added. Then 5 equivalents of sodium Hydride was added at 0 degrees and it was stirred at RT for 2 hours. Quenched the reaction mixture with Ice cold water and extracted with ethyl acetate to get the product. All the synthesized compounds were confirmed by 1H and 13C-NMR spectral data.
General procedure for the synthesis of 2-(2-amino-6-phenyl-4-pyrimidinylamino)ethanol
To the 500mg of Starting material (N,O-ketene derivatives) taken in 10ml of DMSO and 10ml of toluene, two equivalents of guanidine.HCl and 2 equivalents potassium tert. But oxide was added at RT. it was stirred at 1100C for 5 hours. Quenched the reaction mixture with water and extracted with 10% methanol in chloroform to get the product, which was stirred with diethyl ether and filtered to get required product. All the synthesized compounds were confirmed by 1H NMR, 13C-NMR, Mass and IR.
2-(2-amino-6-(2,6-dimethoxyphenyl)pyrimidin-4-ylamino)ethanol
M.P. 1900C; Rf value 0.45 (in 10% Methanol in DCM); H1 NMR (500 MHz, DMSO-d6, 27°C) δ ppm 3.3(2H, bs), 3.5 (2H, bs), 3.64 (6H, s), 4.72 (1H, s, D2O exchangeable), 5.59 (1H, s), 5.82 (2H, s, NH2, D2O exchangeable), 6.65 (2H, d, J=8.5), 6.74 (1H, bs, D2O exchangeable ) 7.26 (1H, t, J=8.5); 13C NMR (400 MHz, DMSO-d6, 250C) δ ppm 42.5, 55.4 , 60.0 , 96.9, 103.9, 118.3, 129.0, 157.1, 159.7, 162.7, 163.1; ESI MS: [M.++H] 291 m/z; IR: 3477cm-1, 3415cm-1, 3332cm-1, 3198cm-1, 2960cm-1, 2929cm-1, 2853cm-1
2-(2-amino-6-(4-methoxyphenyl)pyrimidin-4-ylamino)ethanol
M.P. 1480C; Rf value 0.50 (in 10% Mein DCM); 1H NMR (300MHz, DMSO) δ ppm 3.3 (2H, t), 3.4 (2H, t, J=5.4), 3.77 (3H,s),4.71 (1H, t, J=5.1, D2O exchangeable), 5.90 (2H, s, D2O exchangeable), 6.18 (1H,s),6.76 (1H, S, D2O exchangeable), 6.96 (2H,d, J=8.7), 7.83 (2H,d,J=5.4); 13C NMR (400 MHz, DMSO-d6, 250C) δ ppm 42.7, 55.1, 60.1, 89.9, 113.6, 127.5, 130.6, 160.2, 163.2 , 164.1; IR: 3443cm-1, 3315cm-1, 3218cm-1, 2987cm-1, 2956cm-1, 2937cm-1, 2862cm-1
2-(2-amino-6-phenylpyrimidin-4-ylamino)ethanol
M.P. 1180C; Rf value 0.65 (in 10% Methanol in DCM) H1 NMR (500 MHz, DMSO-d6, 27°C) δ ppm 3.3(2H, t), 3.5 (2H, dd, J=6, J=11.5), 4.72 (1H, t, J=5, Exchangeable proton), 6.02 (2H, s, -NH2, Exchangeable proton), 6.27 (1H, s), 6.95 (1H, bs, Exchangeable proton), 7.49 (2H, d, J=8.5), 7.91 (2H,d, J=7.5); 13C NMR (400 MHz, DMSO-d6, 250C) δ ppm 42.7, 60.0, 90.9, 126.1, 127.2, 128.2, 128.9, 129.2, 138.3, 161.4, 163.4, 164.1; ESI MS: [M.++H] 231 m/z; IR: 3386cm-1, 3325cm-1, 3221cm-1, 3110cm-1, 2992cm-1, 2938cm-1, 2879cm-1
2-(2-amino-6-(4-chlorophenyl)pyrimidin-4-ylamino)ethanol
M.P. 1780C; Rf value 0.49 (in 10% Methanol in DCM); H1 NMR (500 MHz, DMSO-d6, 27°C) δ ppm 3.3 (2H, t, J=5.4), 3.50-3.53 (2H, m),4.73 (1H, t, J=5, Exchangeable proton), 5.97 (2H,s, -NH2, exchangeable proton), 6.26 (1H, S), 6.87 (1H,bs, exchangeable proton), 7.39-7.45 (3H,m), 7.99 (2H, d, J=6.5); 13C NMR (400 MHz, DMSO-d6, 250C) δ ppm 42.7, 59.9, 67.4, 72.5 , 90.95, 127.9, 128.1, 128.3, 133.9, 137.1, 160.1, 163.3, 164.1. ESI MS: [M.++H] 265 m/z; IR: 3403cm-1, 3317cm-1, 3217cm-1, 3104cm-1, 2951cm-1, 2924cm-1, 2868cm-1
2-(2-amino-6-(4-fluorophenyl)pyrimidin-4-ylamino)ethanol
M.P. 158-1590C, Rf value 0.47 (in 10% Methanol in DCM) H1 NMR (500 MHz, DMSO-d6, 27°C) δ ppm 3.3 (2H, t, J=5.4), 3.51 (2H, t, J=5.9), 5.99 (2H, S, -NH2, Exchangeable proton), 6.25 (1H,s), 6.90 (1H,bs, Exchangeable proton), 7.26 (2H, t, J=8.8), 7.94 (2H,s); 13C NMR (400 MHz, DMSO-d6, 250C) δ ppm 42.14 , 60.0, 91.0, 115.2, 128.4, 134.8 , 158.3, 160.3, 161.6, 163.3, 164.1, 164.2; ESI MS: [M.++H] 249 m/z; IR: 3398cm-1, 3221cm-1, 3152cm-1, 2994cm-1, 2953cm-1, 2937cm-1, 2879cm-1
2-(2-amino-6-(4-nitrophenyl)pyrimidin-4-ylamino)ethanol
M.P. 1920C, Rf value 0.38 (in 10% Methanol in DCM); H1 NMR (300 MHz, DMSO-d6, 27°C) δ ppm 3.5 (2H, t), 3.79 (2H, t, J=3),4.91 (2H, s), 5.83 (1H,bs),6.28 (1H, S), 7.97 (1H,d), 8.08 (2H, d, J=4.5), 8.26 (2H,d,J=4.8); 13C NMR (400 MHz, DMSO-d6, 250C) δ ppm 42.1, 59.8, 94.9, 123.6, 123.3, 144.6, 147.8, 163.5, 163.8, 164.2; ESI MS: [M.++H] 275 m/z; IR: 3441cm-1, 2954cm-1, 2918cm-1
(E)-1-(4-methoxyphenyl)-2-(oxazolidin-2-ylidene)ethanone (Compound 3b): H1 NMR
(300 MHz, DMSO-d6, 27°C) δ ppm 3.70 (2H, t, J=8.4), 3.78 (3H, s), 4.45(2H, t, J=8.4), 5.50 (1H, s), 6.93 (2H, d, J=8.7), 7.79 (2H, d, J=8.7), 9.72 (1H, bs); C13 NMR (400MHz, DMSO-d6, 250C) δ ppm 43.015, 55.501, 67.0, 67.2, 72.0, 113.2, 128.2, 128.2, 132.5, 161.0, 169.3, 184.2.
(E)-2-(oxazolidin-2-ylidene)-1-phenylethanone (compound 3c): H1 NMR
(400 MHz, DMSO-d6, 25°C) δ ppm 3.72 (2H, t, J=8.4), 4.46 (2H, t, J=8.4), 5.55 (1H, s), 7.41 (3H, m), 7.83 (2H, d, J=6.4), 9.81 (1H, bs); C13 NMR (400MHz, DMSO-d6, 250C) δ ppm 46.8, 67.3, 72.5, 126.4, 128.3, 133.2, 139.9, 169.6, 184.7.
(E)-1-(4-chlorophenyl)-2-(oxazolidin-2-ylidene)ethanone (compound 3d): H1 NMR
(400 MHz, DMSO-d6, 25°C) δ ppm 3.72 (2H, t, J=8.4), 4.47 (2H, t, J=8.4), 5.53 (1H, s), 7.42 (2H, d, J=8.4), 7.83 (2H, dd, J=8.8, J=2.0), 9.81 (1H, bs); C13 NMR (400MHz, DMSO-d6, 250C) δ ppm 43.0, 67.4, 72.5, 128.0, 128.3, 135.0, 138.6, 169.6, 183.2.
(E)-1-(4-fluorophenyl)-2-(oxazolidin-2-ylidene)ethanone (compound 3e): H1 NMR
(400 MHz, DMSO-d6, 25°C) δ ppm 3.72 (2H, t, J=8.4), 4.469 (2H, t, J=8.4), 5.53 (1H, s), 7.19 (2H, d, t=8.8), 7.87 (2H, m), 9.76 (1H, bs); C13 NMR (400MHz, DMSO-d6, 250C) δ ppm 43.054, 67.3, 72.3, 114.7, 114.9, 128.8, 128.9, 136.4, 136.4, 162.2, 164.6, 169.6, 183.4.
(E,Z)-1-(4-nitrophenyl)-2-(oxazolidin-2-ylidene)ethanone (compound 3f as a mixture of E,Z isomers in 10:1 ratio): H1 NMR
(400 MHz, DMSO-d6, 25°C) δ ppm 3.71 (0.2H, t, J=8), 3.75 (2H, t, J=8.8), 4.45 (0.2H, t, J=8.4), 4.51 (2H, t, J=8.8), 5.51 (0.1H, s), 5.61 (1H, s), 7.25 (0.2H, dd, J=2, J=6.8), 7.75 (0.2H, dd, J=2, J=6.8), 8.03-8.06 (2H, m), 8.21-8.24 (2H, m), 9.76 (0.1H, bs), 9.92 (1H, bs); C13NMR (400MHz, DMSO-d6, 250C) δ ppm 43.0, 43.1, 67.6, 73.6, 123.3, 124.8, 127, 127.7, 145.4, 148.3, 169.8, 182.0.
Conclusion
Six novel pyrimidine derivatives (4a to 4f) were prepared and characterized by IR, Mass, 1H and 13C-NMR spectral data. In conclusion, we have successfully synthesized 2-(2-amino-6-aryl-4-pyrimidinamino) ethanol Derivatives. The molecular docking studies for the synthesized compounds revealed that all the synthesized compounds best fit into the active site of HDAC2. Compound 4c, 4d, 4f is showing better results.
Acknowledgements
The authors expressed their gratitude to Dr. M. Mallika, Department of Medicinal Chemistry, NIPER-Hyderabad, for providing docking facility for the present research work.
References
- Katiyar, Sanjay Babu et al; Bioorganic & Medicinal Chemistry Letters 2005, 15(1), 47-50;
CrossRef - Rajani Giridhar, Riyaj S. Tamboli, Dhaval G. Prajapati, Sanket Soni, Sarita Gupta, M. R. Yadav; Med Chem Res 2013, 22, 3309–3315.
CrossRef - Scobie, Martin; Wallner, Olov; Koolmeister, Tobias; Vallin, Karl Sven Axel; Henriksson, Carl Martin; Jacques, Sylvain; Homan, Evert; Helleday, Thomas; PCT Int. Appl. WO 2015187088 A1, 2015.
- Love, Christopher John et al; PCT Int. Appl. 2003015776, 27 Feb 2003.
- Cao, Sheldon Xiaodong et al; PCT Int. Appl. 2002096867, 05 Dec 2002.
- Daniela Cortese, Konstantin Chegaev, Stefano Guglielmo, Lan Z. Wang, Bernard T. Golding, Cline Cano and Roberta Fruttero; ChemMedChem 2016, 11, 1705-1708.
CrossRef - Potts, K. T.; Cipullo, M. J.; Ralli, P.; Theodoridis, G.; J. Org. Chem. 1982, 47(9), 3027;
CrossRef - Junjappa, H.; Ila, H.; Asokan, C. V.; Tetrahedron 1990, 10, 5423;
CrossRef - Rudorf, W. D.; Schierhorn, A.; Augustin, M.; Tetrahedron 1979, 35, 551.
CrossRef - Yingquan Song, Hondamuni I. De Silva, William P. Henry, Guozhong Ye, Sabornie Chatterjee, Charles U. Pittman Jr.; Tetrahedron Letters 2011, 52, 4507–4511.
CrossRef - Singh, O. M.; Ila, H.; Junjappa, H.; Indian Journal of Chemistry – Section B: Organic and Medicinal Chemistry, 1997, 36 (12), 1123-1125.
- National Committee for Clinical Laboratory Standards 1993a. Performance Standards for Antimicrobial Disk Susceptibility Tests—Fifth Edition: Approved Standard M2-A5. NCCLS,Villanova, PA.
- Friesner, R. A.; Murphy, R. B.; Repasky, M. P.; Frye, L. L.; Greenwood, J. R.; Halgren,T. A.; Sanschagrin, P. C.; Mainz, D. T.; J. Med. Chem. 2006, 49, 6177–6196.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









