Synthesis of a New Cyclen-based Compound as a Potent Anti-tumor Medicine
Davood Habibi1* Hiva Nabavi2, Somayyeh Heydari2, Somayyeh Vakili2
1Department of Chemistry, Faculty of Science, Takestan Islamic Azad University, Takestan, Iran 2Department of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, Hamedan, Iran
DOI : http://dx.doi.org/10.13005/ojc/290317
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
A new cyclen based compound (5a,6,7,8,9,9a,15a,16,17,18,19,19a-dodecahydrodinaphtho[2,3-b:2’,3’-h][1,4,7,10]- tetraazacyclododecine-5,10,15,20-tetraone) was successfully synthesized from the reaction of naphthoquinone with ethylenediamine in a phosphate buffer/acetonitrile solution under mild reaction conditions in excellent yield.
KEYWORDS:Naphthoquinone;Ethylenediamine;Cyclen-based Compound;Phosphate buffer/acetonitrile solution;Medicine
Download this article as:| Copy the following to cite this article: Habibi D, Nabavi H, Heydari S, Vakili S. Synthesis of a New Cyclen-based Compound as a Potent Anti-tumor Medicine. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290317 |
| Copy the following to cite this URL: Habibi D, Nabavi H, Heydari S, Vakili S. Synthesis of a New Cyclen-based Compound as a Potent Anti-tumor Medicine. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=290 |
INTRODUCTION
Cyclen is one of the most important ligands in medicinal chemistry and derivatives of this macrocycle are used to form stable metal complexes with gadolinium, copper, indium, gallium, zinc, and other metals. These complexes are used extensively in medical imaging and radiotherapy.1 Also, cyclen has strong coordination ability toward a wide range of cations and their complexes have been widely used in DNA recognition and cleavage as chemical nucleases.2-6 Research concerning polymeric materials containing the tetraazacyclododecane structure is of growing interest and has found applications in medicine.7,8
Cyclen (1,4,7,10-tetraazacyclododecane), cyclam (1,4,8,11-tetraazacyclotetradecane) and their derivatives have been studied as carrier of metal ions in anti-tumor9-13 and imaging applications14-16 and as anti-HIV agents.17-18
In continuation of our studies on the chemical behavior of different catechols in the presence of different diamines and synthesis of various heterocycles,19-28 we would like to report the synthesis of 5a,6,7,8,9,9a,15a,16,17,18,19,19a-dodecahydrodinaphtho[2,3-b:2’,3’-h][1,4,7,10]- tetraazacyclododecine-5,10,15,20-tetraone from the reaction of p-naphthoquinone and ethylenediamine in in ice bath phosphate buffer/acetonitrile solution (Scheme 1) in excellent yield.
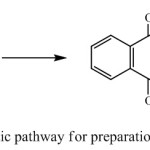 |
Scheme 1: Synthetic pathway for preparation of target molecule Click here to View figure |
RESULTS AND DISCUSSIONS
Since cyclen and its derivatives have been used as medicines, we were interested to synthesize some new cyclen based compound as a potent anti-tumor medicine. So in the first step, 5a,6,7,8,9,9a,15a,16,17,18,19,19a-dodecahydrodinaphtho[2,3-b:2’,3’-h][1,4,7,10]tetraazacyclo- dodecine-5,10,15,20-tetraone was synthesized from the reaction of p-naphthoquinone and ethylenediamine in a phosphate buffer/acetonitrile solution at 0 ºC in excellent yield. In the second step and after separation and purification of the product, it was characterized with different spectroscopic methods (IR, NMR and MASS). The main peak at m/z 432 in the mass spectrum and data obtained from the IR and NMR, definitely confirms synthesis of the target molecule.
Different conditions were used to optimize the reaction condition. In the reflux or in room temperature, oily materials were produced which their characterizations were not easy (may be due to the polymer formation), so the reaction was carried out in 0 ºC.
Also, in the basic pHs, the OH¯ might compete as a nucleophile with ethylene diamine. In addition, the acidic pHs will decrease the nucleophilic power of ethylene diamine, so we preferred to do the reaction in neutral buffer solution (pH = 7).
The plausible mechanism of the reaction has been illustrated in Scheme 2. According to this mechanism, ethylene diamine will attack as a nucleophile to naphthoquinone (1a) to produce 1b, and the subsequent nucleophilic attack of 1b to 1a will produce 2b. Hydrogen abstraction of 2b in the presence of ethylene diamine (as a base) will produce 3b. Another nucleophilic attack of ethylene diamine to 3b will give 4b which the internal attack of amino group of 4b will produce 5b.
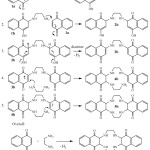 |
Scheme 2: A plausible mechanism for the synthesis or a cyclen based compound Click here to View figure |
EXPERIMENTAL
Reagents and chemicals
All reagents and chemicals were purchased from the Merck and Aldrich chemical companies and used without further purification. 1H NMR spectra were recorded on a Bruker Avance DRX 300 MHz instrument in DMSO. The chemical shifts (d) are reported in ppm relative to TMS as an internal standard. Mass spectra were recorded on a Shimadzu GC-MS QP 1100 EX mass spectrometer. FT-IR (KBr) spectra were recorded on a Perkin-Elmer 781 spectrophotometer. Melting points were taken in open capillary tubes with a BUCHI 510 melting point apparatus and were uncorrected. TLC was performed on silica gel polygram SIL G/UV 254 plates.
Preparation of a phosphate buffer solution
Phosphate buffer solution was prepared according to the Henderson-Hasselbalch equation (pH = pKa + log ([A–]/[HA])). The pKa of sodium dihydrogen phosphate is about 7.2 and since we would like to work in a pH = 7, so a buffer solution was prepared by dissolving NaH2PO4 (4.6785 g, 40 mmol) and Na2HPO4 (8.5815 g, 60 mmol) in water (500 mL).
Typical procedure for the synthesis of a cyclen-based compound
To 1,4-naphthoquinone (0.158 g, 1 mmol) in ice bath phosphate buffer/acetonitrile solution (50:50, 10 ml), was gradually dropwise added ethylenediamine (0.18 ml, 3 mmol) and stirred for 2 h. The color changed very fast and the reaction monitored with TLC. The reaction mixture was filtered, the solvent removed and the filtrate washed with diethyl ether (5×10 ml) and purified with column chromatography (acetone/n-hexane 70:30). The orange powder was obtained in 95 % yield, and characterized with different spectroscopic methods (IR, NMR and MASS).
M.p.: 274-276.5 ºC
IR (KBr): 3344, 3045, 2947, 1681, 1640, 1615, 1592, 1561, 1498, 1357, 1329, 1285, 1252, 929, 845, 791, 724 and 581 cm-1.
1H NMR (300 MHz, aceton-d6): δ 2.0 (4 NH), 3.36 (4 CH2), 4.08 (4 CH) and 8.0 (8H, aromatic).
13C NMR (125 MHz, DMSO-d6): δ 40 (C of CH2), 79 (C of CH), 122-145 (Cs aromatic) and 150 (C of carbonyl).
MASS (M+): 432
CONCLUSION
We were able to synthesize a cyclen based compound (5a,6,7,8,9,9a,15a,16,17,18,19,19a-dodecahydrodinaphtho[2,3-b:2’,3’-h][1,4,7,10]tetraazacyclododecine-5,10,15,20-tetraone) from the reaction of p-naphthoquinone and ethylenediamine in a phosphate buffer/acetonitrile solution under mild reaction conditions in excellent yield.
ACKNOWLEDGEMENT
Financial support from the Takestan Islamic Azad University, as well as the Bu Ali Sina University, Hamedan 6517838683, Iran, is gratefully acknowledged.
REFERENCES
- Anderson, C.J. and Welch, M.J. Chem. Rev., 99: 2219 (1999).
- Kimura, E.; Kitamura, H.; Ohtani, K. and Koike, T. J. Am. Chem. Soc., 122: 4668 (2000).
- Kikuta, E.; Murata, M.; Katsube, N.; Koike, T. and Kimura, E. J. Am. Chem. Soc., 121: 5426 (1999).
- Aoki, S.; Kagata, D.; Shiro, M.; Takeda, K. and Kimura, E. J. Am. Chem. Soc., 126: 13377 (2004).
- Aoki, S.; Iwaida, K.; Hanamoto, N.; Shiro, M. and Kimura, E. J. Am. Chem. Soc., 124: 5256 (2002).
- Aoki, S.; Kawatani, H.; Goto, T.; Kimura, E. and Shiro, M. J. Am. Chem. Soc., 123: 1123 (2001).
- Aime, S.; Botta, M; Crich, S.G., Giovenzanna, G.; Palmisano, G. and Sisti, M. Bioconjugate Chem., 10: 192 (1999).
- Lewis, M.R.; Raubitschek, A. and Shively, J.E. Bioconjugate Chem., 5: 565 (1994).
- Kong, D.-Y.; Meng, L.-H.; Ding, J.; Xie, Y.-Y. and Huang, X.-Y. Polyhedron 19: 217 (2000).
- Liang, F.; Wu, C.-T.; Lin, H.-K.; Li, T.; Gao, D.-Z.; Li, Z.-Y.; Wei, J.; Zheng, C.-Y. and Sun, M.-X. Bioorg. Med. Chem. Lett., 13: 2469 (2003).
- Liang, F.; Wang, P.; Zhou, X.; Li, T.; Li, Z.-Y.; Lin, H.-K.; Gao, D.-Z.; Zheng, C.-Y. and Wu, C.-T. Bioorg. Med. Chem. Lett., 14: 1901 (2004).
- Parker, L.L.; Lacy, S.M.; Farrugia, L.J.; Evans, C.; Robins, D.J.; O’Hare, C.C.; Hartley, J.A.; Jaffar, M. and Stratford, I.J. J. Med. Chem., 47: 5683 (2004).
- Parker, L.L.; Anderson, F.M.; O’Hare, C.C.; Lacy, S.M.; Bingham, J.P.; Robins, D.J. and Hartley, J.A. Bioorg. Med. Chem., 13: 2389 (2005).
- Liu, S. and Edwards, D.S. Bioconjugate Chem., 12: 7 (2001).
- Fichna, J. and Janecka, A. Bioconjugate Chem., 14: 3 (2003).
- Jang, Y.-H.; Blanco, M.; Dasgupta, S.; Keire, D.A.; Shively, J.E. and Goddard, W.A., III J. Am. Chem. Soc., 121: 6142 (1999).
- De Clercq, E. Nat. Rev. Drug Disc., 2: 581 (2003).
- Liang, X.-Y. and Sadler, P.J. Chem. Soc. Rev., 33: 246 (2004).
- Habibi, D.; Nematollahi, D. and Seyyed Al-Hoseini, Z. Electrochim. Acta 52: 1234 (2006).
- Habibi, D.; Mahmoodi, N. and Marvi, O. Synth. Commun., 37: 3165 (2007).
- Habibi, D.; Nematollahi, D. and Azimi, S. Tetrahedron Lett., 49: 5043 (2008).
- Nasrollahzadeh, M.; Habibi, D.; Shakarami, Z. and Bayat, Y. Tetrahedron 65: 10715 (2009).
- Habibi, D.; Nasrollahzadeh, Faraji, A. and Bayat, Y. Tetrahedron 66: 3866 (2010).
- Habibi, D.; Nasrollahzadeh, M. and Kamali, T.A. Green Chem. 13: 3499 (2011).
- Habibi, D. and Nasrollahzadeh, M. Monatsh Chem., 143: 925 (2012).
- Habibi, D.; Nasrollahzadeh, M.; Sahebekhtiari, H. and Sajadi, S.M. Synlett 2795 (2012).
- Habibi, D.; Faraji, A. and Gill, A. Sensors Actuat. B-Chem., 182: 80 (2013).
- Habibi, D.; Nasrollahzadeh, M.; Sahebekhtiari, H. and Parish, R.V. Tetrahedron 69: 3082 (2013).

This work is licensed under a Creative Commons Attribution 4.0 International License.









