Studies on Cu(Ii), Co(Ii), Ni(Ii) and Mn(Iii) Complexes of Merrifield Resin Supported Ligand and Antimicrobial Activities of This Novel Schiff Base Chelates
K. Sankar1 and A. Nazeera Beevi2
1Research Department of Chemistry, M. G. College, Thiruvananthapuram - 695 004 India. 2Department of Chemistry, Government College, Kariyavattom, Thiruvananthapuram - 695 581 India.
The Present paper deals with the study of synthesis and characterisation of Cu(II), Co(II), Ni(II) and Mn(III) complexes of polystyrene with 2-aminobenzaldehyde phenyl hydrazone ligand. The study is supported by magnetic susceptibility, infrared and UV-visible spectral study and thermal studies. The ligand and its metal complexes were screened for their antimicrobial activity using S.aureus and E.coli micro organism and found to be moderately active. All the complexes catalyse the decomposition of H2O2, copper(II) complex being the most active.
KEYWORDS:Merrifield resin; polystyrene; 2-aminobenzaldehyde phenyl hydrazone; Schiff base; antimicrobial activity
Download this article as:| Copy the following to cite this article: Sankar K, Beevi A. N. Studies on Cu(Ii), Co(Ii), Ni(Ii) and Mn(Iii) Complexes of Merrifield Resin Supported Ligand and Antimicrobial Activities of This Novel Schiff Base Chelates. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Sankar K, Beevi A. N. Studies on Cu(Ii), Co(Ii), Ni(Ii) and Mn(Iii) Complexes of Merrifield Resin Supported Ligand and Antimicrobial Activities of This Novel Schiff Base Chelates. Orient J Chem 2011;27(2). Available from: http://www.orientjchem.org/?p=25079 |
Introduction
Among the different polymer supported metal complexes, Schiff base polymer metal complexes 1,2 are important. Apart from their novel structural features, unusual magnetic properties and role in biological processes, the polymer supported Schiff base metal complexes are important in preconcentration 3,4 of metal ion, catalysis 5,6 etc
The field has grown so wide ever since Schiffbases7 were synthesized. With a preformed metal complex of compound containing a carbonyl group, Pfeitter 8,9 and coworkers continued the study of Schiff base complexes. One of the first chelates of a Schiff base to be isolated was bis (salicylaldimino) copper (II), prepared by the reaction of copper(II) acetate with salicylaldehyde and aqueous ammonia. Synthesis of polymer complexes using bis-bidentate Schiff base ligands were studied and their structural elucidation are done already10, 11. A number of polymer Schiff base ligand has been synthesized for their ion-selective properties 12,13.
The present work deals with synthesis and characterization of polymeric copper(II), nickel(II), cobalt(II) and manganese(III) complexes of Schiff base derived by condensation of Merrifield resin with 2-amino benzaldehyde phenyl hydrazone. The complexes have been characterised by various physico chemical methods.The catalytic activity of the complexes was also studied. The antimicrobial activity of the ligand and its metal chelates against S. aureus and E.coli has also been studied.
Experimental
All the chemicals used were of AR grade. Merrifield resin (Fluka) and 2-amino benzaldehyde phenyl hydrazone(Fluka) were used as such. Metal salt solutions were prepared in doubly distilled water.
The resin and metal complexes were characterized by analytical and spectral methods.The spectral methods employed were IR and UV-vis spectroscopy.The IR spectra were taken on a Perkin Elmer FTIR Paragon 1000 spectrophotometer (4000-400cm-1). UV –visible spectra were recorded on a Schimadzu 2100 UV-visible spectrophotometer. Magnetic susceptibility was measured on a Gouy balance using Hg [Co(NCS)4] as the calibrant. Thermal analysis was done on a Dupont 2000 thermo balance in air at a heating rate of 10oC/min. Standard volumetric methods were employed to determine metal ion concentration.
Preparation of the Schiff base ligand
The Schiff base was obtained by the condensation of Merrifield resin (0.7 mmol Cl/g,3g) with 2-aminobenzaldehyde phenyl hydrazone (3.5 mmol,0.8g). 2-aminobenzaldehyde phenyl hydrazone was refluxed with Merrifield resin in THF(15ml) in the presence of pyridine (7 mmol) for about 8 hours to get the ligand. The product was then filtered, repeatedly washed with distilled water and then finally with ethanol and then dried. The reaction is represented in scheme-1.
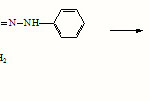 |
Scheme 1 Click here to View Scheme |
Preparation of complexes
0.02M solution of copper(II)acetate,nickel(II)acetate,cobalt(II)acetate and manganese(III) acetate were prepared.A fixed quantity was pipetted out and shaken with the Schiff base (0.5g) for 24 hours. The initial and final concentration of metal solution were determined volumetrically.
Results and Discussions
IR Spectra
The IR spectral data are given in Table 1.Many of the IR Spectral band of the complexes show mark difference in position and intensity from those in the spectrum of the ligand. The spectrum of the ligand shows a band at 3200 cm-1due to NH group and another band at 1580 cm-1 due to azomethine group. In copper(II) complex the NH stretch is shifted to 3180 cm-1 and in the cobalt (II) complex it is 3170 cm-1 and in nickel (II) complex it is 3178 cm-1 and in manganese (III) complex it is 3180 cm-1 and the azomethine vibration is shifted to 1570 cm-1 for copper (II) complex and 1565 cm-1 for cobalt (II) complex, 1568 cm-1 for nickel (II) complex and 1570 cm-1 for manganese(III) complex14. The complexes shows bands due to acetate group around 1560 cm-1 and 1450 cm-1. The gap of 110 cm-1 between these bands show that the acetate group is bidentate in the complexes. Metal-nitrogen15 stretching vibration are observed around 565 cm-1 and metal –oxygen stretches are observed around 595 cm-1.
Table 1: IR Spectral data of ligand and complexes
| N-H(cm-1) | R2-C = N-R(cm-1) | CH3COO–( cm-1) | M-N(cm-1) | M-O(cm-1) | |
| Ligand | 3200 | 1580 | |||
| Copper (II) complex | 3180 | 1570 | 1560 1450 | 565 | 595 |
| Cobalt (II) complex | 3170 | 1565 | 1562 1452 | 563 | 592 |
| Nickel (II) complex | 3178 | 1568 | 1565 1451 | 562 | 591 |
| Manganese (III) complex | 3180 | 1570 | 1563 1453 | 561 | 595 |
Electronic Spectra
The Electronic spectra show a band in the visible region with a maximum around 20830cm-1 and another band around 18520cm-1. The electronic spectral data suggest a distorted octahedral symmetry for the complexes. The values are given in Table 2.
Table 2 : UV-visible spectral data of complexes
| Complex | Band 1 | Band 2 |
| Cu(II) | 20830 | 18520 |
| Co(II) | 20832 | 18523 |
| Ni(II) | 20834 | 18522 |
| Mn(III) | 20831 | 18521 |
Magnetic behaviour
The copper(II), cobalt(II), nickel(II) and manganese(III) complexes shows magnetic moment values 1.63,3.69, 2.80 and 4.72 B.M respectively. The values agree with the expected values.16
Phenomenological data of thermal decomposition of Merrifield resin supported Schiff base complexes.
All the complexes have only one stage of decomposition. The Ti, Tf and Ts values for the above mentioned complexes are given in Table 3. The MF-ABP copper(II) complex was found to be stable up to 613K(Ti), the decomposition takes place from 613K to 781K(Tf) and Ts occurs at 665K. Of these Cu(II) complex shows the maximum Ti and Tf values.
Table 3: The Phenomenological data of thermal decomposition
| Metal Schiff base complexes | Decomposition temperature range in TG(K) | Peak temperature DTG (K) Ts | |
| Ti | Tf | ||
| Cu(II) | 613 | 781 | 665 |
| Co(II) | 573 | 773 | 713 |
| Ni(II) | 593 | 781 | 703 |
| Mn(III) | 611 | 780 | 701 |
Kinetic data of the thermal decomposition of Merrifield resin supported Schiff base chelates.
The kinetic parameters were evaluated using Coats-Redfern equation and the values of E, – log A and –ΔS are shown in Table 4. The negative value of ΔS for the complexes indicates that the activated complex has a more ordered structure than the reactants 17.
Table 4: Kinetic data of thermal decomposition
| Metal ion of Schiff base complex of MF-IA ligand | E
kJ mol-1 |
-log A | -ΔS J K-1 mol-1 |
| Cu(II) | 41.66 | 0.28 | 246.18 |
| Co(II) | 32.13 | 0.67 | 265.01 |
| Ni(II) | 36.49 | 0.68 | 252.52 |
| Mn(III) | 39.68 | 0.59 | 260.12 |
Influence of H2O2 concentration on the rate of decomposition of Schiff base complexes
A definite amount of catalyst (100mg) was subjected to decomposition reaction by varying the concentration of H2O2 (1.8g/L to 3g/L). The result for different complexes is given in Table 5. The rate of reaction is found to increase with increase in concentration of H2O2. Of these copper(II) complex is most active.
Table 5: Influence of H2O2 concentration on the rate of decomposition Amount of catalyst 100mg
| Concentration of H2O2 (g/L) | Rate constant 103kmin-1 | |||
| Cu(II) | Co(II) | Ni(II) | Mn(III) | |
| 1.8 | 4.82 | 4.01 | 4.21 | 4.18 |
| 2.4 | 5.42 | 4.58 | 5.02 | 5.01 |
| 3.0 | 6.38 | 5.32 | 5.98 | 6.01
|
The influence of varying amount of catalyst in the Schiff base complexes of Merrifield resin
The effect of varying amount of catalyst on the decomposition reaction shows first order dependence. For a definite concentration of H2O2 (1.8g/L) varying amounts of catalyst (50mg, 100mg, 150mg, 200 mg) are subjected to decomposition reaction. The rate of reaction increased with increase in amount of catalyst. The values given in Table 6. Here also the Copper(II) complex is found to be the most active.
Table 6: Influence of varying amount of the catalyst on the rate of decomposition Concentration of H2O2 = 1.8g/L
| Amount of Catalyst
(mg) |
Rate constant 103kmin-1 | |||
| Cu(II) | Co(II) | Ni(II) | Mn(III) | |
| 50 | 4.01 | 3.88 | 3.52 | 3.82 |
| 100 | 4.81 | 4.01 | 4.18 | 4.52 |
| 150 | 5.82 | 4.62 | 5.05 | 5.01 |
| 200 | 6.68 | 5.82 | 6.28 | 6.11 |
Antimicrobial screening of the ligand and complexes
Antimicrobial activity of the ligand and its metal chelates has been studied by disc diffusion method18. The activity of the compound was assessed by measuring the diameter of inhibited zone in millimeter (mm). The culture of E.coli bacteria and S.Aureus bacteria were used as test organism, which were grown on nutrient agar medium and gentamycin as control. DMSO was used as solvent for making test solution of all compounds studied. The paper disc (6mm) containing the compound (100µg/disc) was placed on the surface of the nutrient agar plate previously spread with 0.1ml of sterilized culture of microorganism. After incubating this at 37oC for 36 hours, the diameter of inhibition zone around paper disc was measured. The complexes are more active than the ligand. The copper (II) complex has the highest activity against S. Aureus and E.coli. The result are shown in Table 7.
Table 7: Antimicrobial activity of the ligand and complexes
| Compound | S. aureus (zone formation in mm) | E. Coli (zone formation in mm) |
| Control | 24 | 16 |
| MF-ABP ligand | 15 | 12 |
| Ni(II) complex | 16 | 14 |
| Co(II) complex | 18 | 14 |
| Cu(II) complex
Mn(III) complex |
23 | 15 |
| 19 | 13 |
Conclusion
The IR spectral studies of the complexes reveal that the azomethine N and N of NH group are involved in co-ordination. The UV visible spectral study reveals a distorted octahedral structure for the complexes. A tentative structure is shown in figure 1.
The synthesized complexes were evaluated for their antimicrobial and catalytic activities. The antimicrobial activities of the complexes revealed that Copper(II) complex has the highest activity against S.aureus and E.coli.
The catalytic activities of all Schiff base complexes were studied using H2O2 solution. In the case of varying concentration of H2O2,the catalytic activity increases by increasing the concentration H2O2 . The catalytic activity of the complex was also checked by varying the amount of catalyst. Here also the catalytic activity increases by increasing the amount of catalyst.
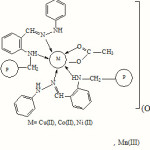 |
Figure 1 Click here to View figure |
Acknowledgement
One of the authors (K.S) gratefully acknowledges the financial support of U.G.C. Bangalore in the form of a Minor Research Project.
References
- J.F. Krebs, A.S. Borovik, Chem. Commun, 5,553 (1998)
- R.Wang, Y.Wang, Lishan Gaofenzi Tongbao,129, CA,33 (1998)
- R. Maya, K. Sreekumar, in Polymer Science, Recent Advances,vol.2,Bharadwaj,Allied Publishers,New Delhi, 985 (1994)
- M.O. Anil Kumar, K. Sreekumar, in Polymer Science, Recent Advances, vol.2, Bharadwaj, Allied Publishers,New Delhi, 997 (1994)
- S. Tangestaninejad, V. Mirkhani, J. Chem Res, 12,788 (1998)
- B.B.De, B.B. Lohre, S. Sivaram, P.K. Dhal J. Polym.Sci,Polym.Chem,ed, 35,1809 (1997)
- H. Schiff, Ann. Chem, 131, 118 (1864)
- P.Pfeitter, E. Bucholz, O. Bauer, J. Prakt.chem, 12,163 (1931)
- P.Pfeitter, H. Knebs, J. Prakt. Chem, 77,155(1940)
- S.R. Aswale, P.R. Mandlik and A.S. AswanIndian J.chem. 42A, 322 (2003)
- A.S. Aswar, S.R. Aswale and P.R. Mandilik, Indian J.chem 43 A, 1892 (2004)
- R.M. Patel, P.T Maskar and M.M Shaikh, Asian J.chem, 19,4563 (2007)
- R.M. Patil, Sivkumar R. Chaursiya, Asian J.chem, 20, 4477 (2008)
- K.Nakamoto,Infrared Spectra of Inorganic and Coordination Compounds.Wiley Interscience, New York(1970).
- L.J.Bellamy,The Infrared Spectra of Metal Complexes,Chapmann and Hall, New York, Vol.2,edn.2(1980)
- F.A.Cotten and M.Goodgame, J.Phys.Chem,65,191(1961).
- V. Indira, G. Parameswaran, Thermochimica, Acta, 101, 145 (1986)
- P.K. Mukherjee, K. Saha, S.M. Giri and B.P Saha, Indian J. Microbiol, 35,327 (1995)

This work is licensed under a Creative Commons Attribution 4.0 International License.









