Adsorption Isotherms 2-aminophenol and 4-aminophenol on Carbon Nonotube
Mehdi Vadi1* and Yalda Ghasemi2
1Department of Chemistry, Fasa Branch, Islamic Azad University, Fasa, Fars (Iran). 2Department of Chemistry, Firouzabad Branch, Islamic Azad University, Firouzabad, Fars (Iran)
Present research paper deals with the adsorption isotherms rate 2-amino phenol and 4-amino phenol on multi-wall carbon nanotube which performed by changing the amount of solution concentration in ppm unit. According to difference between two under studied substances are in substitution of NH2 in ortho and para position , more adsorption of 4-aminophenol on carbon nanotube demonstrate that para position has more adsorption in relation to ortho position .calculation of adsorption isotherms performed by Langmuir, Freundlich and Temkin and relative parameters of each model presented . Taken result in constant temperature laboratory distributes more acceptable identity between data and models, and appears best identity in Freunlich model.
KEYWORDS:Isotherm; carbon nanotube; adsorption; Temkin model; Langmuir model; Freundlich model
Download this article as:| Copy the following to cite this article: Vadi M, Ghasemi Y. Adsorption Isotherms 2-aminophenol and 4-aminophenol on Carbon Nonotube. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Vadi M, Ghasemi Y. Adsorption Isotherms 2-aminophenol and 4-aminophenol on Carbon Nonotube. Orient J Chem 2011;27(2). Available from: http://www.orientjchem.org/?p=24910 |
Introduction
Nanotubes are forms of carbonic compounds with complicated structures that which have longitual strings instead of global structure [1].carbon nanotubes (CNTs) invented at 1991[2].CNTs is much important than fulerns because of it has been from Grafit sheet .Grafit molecular structure is as very tin fiber constitutes from carbonic aromatic.usal Grafit structure is layers of carbon rubbing on together easily.
Relation of adsorption rate variability to concentration of remained amount in solution at constant temperature called adsorption isotherm [3].adsorption isotherms curve has different shapes for variety system. With accordance to prior performed experiments, three equations exist for relation of sorts’ adsorption on different absorbance [4].by test performance for each distinct system; give insight that which of three equation can be used as a adsorption isotherm. In this relations and equations,tempratur of system should be maintained constantly in durance of experiment, because of it called adsorption isotherms, we can determine amount of absorbance in per second with using adsorption isotherm[5].
Material and methods
Material
Ethanol %96 as dissolver, multi-wall carbon nanotube and 2-amino phenol and 4-amino phenol was purthased from Merck co at Germany.
Methodes
A stock solution of about 100mg/L 2-Amino phenol and 4-Amino phenol was prepared. The range of 2-Amino phenol and 4-Aamino phenol concentration used is from 2 to10 mg/L.Equilibrium adsorption experiments were performed using 50ml screw-capped glass centrifuge tubes as batch reactor systems. Each tube containing 0.01mg CNTs was filled with 10ml 2-Amino phenol and 4-Amino phenol solution of different concentrations. All tubes were immediately sealed with PTFE-lined caps and were then mechanically shaken for 24h in a thermostated rotary shaker at temperature of 293K.
Results and discussion
Different models using for adsorption expression. We using on the isotherms Langmuir,Freundlich and Temkin.
Langmuir model
This model of adsorption is used more ordinary and is expressed by equation (1).
![]()
In this equation, qe (mg.g-1) is amount of adsorbed material in absorbent surface and qm is equilibrium constant of adsorption and b is the capacity of adsorption in saturated single layer and Ce (mg.L-1) is solution in equilibrium state.
Freundlich model
This model is on experiential equation which is used more for comprehension of metallic ions adsorption upon heterogeneous surface with multi layer adsorption and also for adsorptions that the adsorption amount is increased unlimited with increasing intension. This model is specified with equation (2).
![]()
In this equation, qe (mg.g-1) is amount of adsorbed material in absorbent surface, K, n in arrangement are adsorption capacity and adsorption intensification.
Temkin model
Temkin isotherm presents the reaction between adsorbent and absorbed particles clearly. This model is specified with equation (3).
![]()
In this relation, A is equivalent of bond constant with maximum of connect energy, b is constant of Temkin isotherm and B is relevant with adsorption temperature.
Isotherms of adsorption trend on carbonic nano tube (CNT) have been presented in figures 1-6 and this models parameters accounted also it shown in table1.
Table1: Parameters of Langmuir,,Freundlich and Temkin
|
Temkin |
Freundlich |
Langmuir |
||||||||
|
R2 |
b(j/mol) |
B |
A(lit/mg) |
R2 |
Kf(mg/g) |
n |
R2 |
qm(Kg/g) |
b(lit/mg) |
|
|
9238/0 |
74/9 |
465/2 |
463/0 |
9397/0 |
3-10*8/3 |
27/0 |
6083/0 |
092/0- |
16/0- |
2-Amino phenol |
|
9637/0 |
32/15 |
5673/1 |
534/0 |
979/0 |
048/0 |
506/0 |
704/0 |
667/0- |
11/0- |
4-Amino phenol |
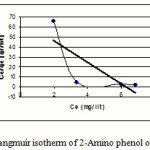 |
Figure 1: Langmuir isotherm of 2-Amino phenol on MW-CNT |
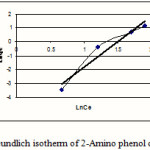 |
Figure 2: Freundlich isotherm of 2-Amino phenol on MW-CNT |
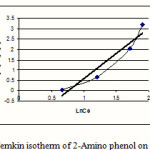 |
Figure 3: Temkin isotherm of 2-Amino phenol on MW-CNT |
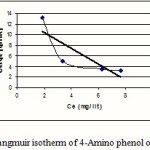 |
Figure 4: Langmuir isotherm of 4-Amino phenol on MW-CNT |
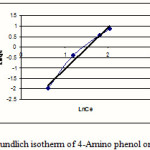 |
Figure 5: freundlich isotherm of 4-Amino phenol on MW-CNT |
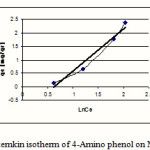 |
Figure 6: temkin isotherm of 4-Amino phenol on MW-CNT |
Click here to View Figure
Conclutions
This research indicates that both under studied organic base showed more adsorption in 10mg/L (10ppm) concentration. According to table1 and 2 observe that usually adsorption of 4-Amino phenol more than 2-Amino phenol, because of lesser spatial repulsion of 4-Amino phenol . in expressed isotherms indicates that adsorption of both organic bases with more value of R2 has an identity with Freundlich model.
References
- H.Kroto, J.Heath,S.O’ Brien , R.Curl and R. Smalley , Nature 318,1985,162.
- S.Lijma, T.Lchihashi, Nature, 363(6430),1993.
- G.J.Clary and M.R.Falo, Carbon nanotube the toutr toward applications.sience, 297, 2002, 787
- K.Yang, W.wu, Q.Jing, L.Zhu, Aqueous adsorption of aniline , phenol and their substitutes by multi walled carbon nanotubes , environ.
- Ruthven, D.John Wiley and Sons, 1984.

This work is licensed under a Creative Commons Attribution 4.0 International License.









