A Fast Route for the Synthesis of Cyclic Guanidine Derivatives
Sudhir Kumar1, Dinesh kumar2 and Renu2
1Department of Chemistry, L.R. (P.G.) College, Sahibabad - 201 005 (India).
2Department of Chemistry, D.A.V, College, Muzaffarnagar - 251 001 (India).
A simple synthesis of cyclic guanidine derivatives is presented in this study. The construction of the central basic scaffolds is achieved by the application of microwave-assisted chemistry. This process completed without use of any extra reagent or protecting group manipulations. The formation of products of substituted guanidine was controlled by the temperature.
KEYWORDS:Guanidine; Guanylation; Diaminoarylether; Amoniumthiocynate
Download this article as:| Copy the following to cite this article: Kumar S, kumar D, Renu R. A Fast Route for the Synthesis of Cyclic Guanidine Derivatives. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Kumar S, kumar D, Renu R. A Fast Route for the Synthesis of Cyclic Guanidine Derivatives. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=24806 |
Introduction
Guanidines is a key structural element of many biological active compounds such as arginine (A small group of about 20 amino acids commonly found in protein).1 It is a basic unit found in the compound which is used for the treatment of many disease such as cancer, diabetic2, metabolic process, guanidine itself is a strong base (pka of conjugated acid is 12.5).4 It was first prepared by streaker in 1861 by oxidizing guanine.5 Guanidine is also used to modulate solubility or to pick up electrostatic interaction6.Cyclic guanidine play an important role in medicine chemsitry7as cataly. sinc Bernal (1951) first suggested that minerals biomolecules as well se as their reactions for the formation of biopolymers. The adsorption of biomolecules on minerals was studied by several author8 .Thiourea is the basic requirment for the synthesis of guanidine in our proposal,9 reported the reaction of protected 1,3-diaminopropane-2-ol with derivatives. The resulting diamino ether was cyclised to the corresponding thiourea with carbon disulphide and carbodiamide10 11. The thiourea was finally transformed in two thermal reactions to the guanidine.
After successfully obtaining the building blocks we set about preparing the thiourea substrate according to the literature12 13, a dithiocarbamate is formed spontaneously when 1 or 2 amine are treated with CS2. Cyclisation can then be achived. This method is to good become it needed high temperature than boiling point of solvent so, it is good for microwave synthesis. Then in microwave cyclic thiourea was obtained as given
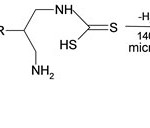 |
Scheme 1 Click here to View scheme |
No intermolecular oligo-or polymerization could be observed.Turning to the synthesis of unsubstantiated guanidine, isothioureonium salts also can be synthesized by this method.Taking alkyl diamine with cyanogenbromide14 15 and NH3.EtOH (Basic medium) and passed it through the microwave for a long time. Give a six member ring system it take 10 hrs for completion and gives 60% yield.In a initial effort to prepare substituted guanidine, we applied the procedure described in the literature.16
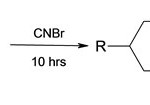 |
Scheme 2 Click here to View scheme |
3b1 R = 4 Chlorophenoxy
3C, R = 3,4-dichlorophenyl
When primary amine were treated with cyclic thiouranium salt in acetonitrile for a very long time (8 days) in microwave region.
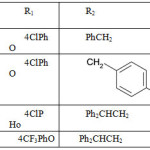 |
Table 1 Click here to View table |
General Procedure for the Guanylation
The starting thiourea, the amine (1,1 equiv), and triethylamine (2.2 equiv) were dissolved in dimethyl formamide (5 ml/mmol substrate) at rt. The mixture was cooled in an ice bath. Mercury (II) chloride (1.1. eq. iv) was added and the mixture and stirred for 20 min, before it was warmed to rt. When the reaction was judged complete (TLC), the reaction mixture was diluted with ethyl acetate and filtered through Celite, washing the Celite cake with additional ethyl acetate. The filtrate was washed with warer, then with brine, and the organic phase was dried with MgSO4. The crude product thus obtaine was purified by flas chromatography on a silica column.1
-N(2)-cyclohexyl-N(3)-phenyl-guanidine
The guanylation was carried out following the general procedure. The title compound was obtained in 70% yield after flash chromatographic purification with ethyl acetate-hexane (1:9) 1H NMR (CDCl3) 7.49-6.96 (5H,m) 6.5 (1H, br, s), 4.73 (1H,br), 3.99 (1H,m), 2./15-0.98 (10H,m), 1.50 ((H,s) IR 3350(w), 2931(m), 16361(m), 1725(m), 1636(m), 1593(s), 1545(m) cm-1, MS m/e 318 (NH+), 262 (100%).
Representative Experimental Procedures for Preparation of guanidine from cyanogen bromide (1.2 mmol, 127 mg) in 2(3a) propanol (2.5 mL) was added 2b (1.0 mmol, 200 mg) and the reaction mixture was heated in the microwave at 120C for 600s. Isopropyl ether (2.5 mL) was added and the precipitate was filtered off, washed with isopropyl ether, and dried in vacuo over P4O10 to give (3a) as a white solid. Yield 61%, 1H NMR (400 MHz, DMSO-d6) 7.68 (br, s, NH, 2H), 7.36 (d, J = 9.0 HZ, 1H), 7.05 (d, J = 9.0 Hz, 1H), 6.95 (br, S,NH, 1H) 4.93 (mc, 1H), 3.44 (mc, 4H); 13C NMR (100 MHz, DMSO-d6) 155.4, 153.7, 129.9, 125.7, 118.3, 65.9, 41.6 ESI-MS: m/z (5) (226 (100) [M + H+].
Preperation of gunidine derivatives using amonium thiocynates
When the results obtained with sample of ammonium thiocyanate were compared to the samples of ammonium thiocyanate (at 120 ºC) plus salts of seawater or transition metals also an increased on the production of thiourea (168%) and guanidine (268%) was obtained, respectively. The yield for the synthesis of guanidine showed in this paper is better than that obtained by other authors. Experiments showed that iron (III) is involving in the formation of the yellow compound and Raman spectra showed this compound could be sulphur. high yields (40-85%) of 2,4-diaminopyrimidine
pointed out the importance of cyanoguanidine as a condensing agent in phosphorylation and peptide formation. Guanidine has also important roles to play in modern living beings.In spite of the importance of guanidine in prebiotic chemistry as well as the modern living being, as far as we know there are two papers about prebiotic synthesis of guanidine. Halmann17 reported that cyanamide solution converts to cyanoguanidine and Lohrmann18 studied the formation of urea and guanidine by irradiation of ammonium cyanide.Ammonium and thiocyanate have been synthesized in experiments under prebiotic chemistry conditions or have been found in places that resembling these environments. Dowler and Ingmanson19 found thiocyanate in Atlantis II Deep Brine, located at the bottom of the sea in the Rift Valley Summers20 showed that nitrite could be reduced to ammonia by iron (II) in the oceans. A review paper, published by Raulin and Toupance, shows that ammonium thiocyanate was formed when a primitive atmosphere (CH4, NH3, H2O, H2, N2, CO2, H2S) and electric discharge as energy source were used. So, ammonium and thiocyanate are substances that easily would be present on the primitive Earth.In this paper we describe the synthesis of guanidine from the reaction of ammonium thiocyanate in solid state with different temperatures and several substances.
Samples of ammonium thiocyanate and ammonium thiocyanate plus river sand or kaolin. Two sets with two samples each were always run. First set: each sample with about 3.0 g of ammonium thiocyanate plus 5.0 g of river sand or kaolin was weighted and mixed until a homogenized was obtained. Second set: each sample with about 3.0 g of ammonium thiocyanate was weighted. Aliquots were withdrawn for determination of concentration of ammonium and thiocyanate as a zero time. The samples were added to a sublimation apparatus and heated for 3 days at different temperatures (80, 120, 150 ºC). After heating, the crude sublimate and sample at the bottom were removed from sublimation apparatus and mixed. Aliquots were withdrawn and analysis of ammonium, thiocyanate, thiourea, and guanidine thiocyanate were carried out as described in methodology.
Samples of ammonium thiocyanate plus salts of seawater and ammonium thiocyanate plus salts of seawater plus river sand. Two sets with two samples each were always run. First set: each sample with about 3.0 g of ammonium thiocyanate plus 5.0 g of river sand plus 5.0 g of salts of seawater was weighted and mixed until a homogenized was obtained. Second set: each sample with about 3.0 g of ammonium thiocyanate plus 5.0 g of salts of seawater was weighted and mixed until a homogenized was obtained. Aliquots were withdrawn for determination of concentration of thiocyanate as a zero time. The samples were added to a sublimation apparatus and heated for 3 days at 120 ºC. After heating, crude sublimate and sample at the bottom were removed from the sublimation apparatus and mixed. Aliquots were withdrawn and analysis of thiocyanate, thiourea, and guanidine thiocyanate were carried out as described in methodology.
General method
Before each run, the sublimation apparatus was shaked with hot concentrated sulfuric acid to oxidize any organic substance. After, the sublimation apparatus was washed out with plenty of distilled water to remove the sulfuric acid. Then, It was dried at 200 ºC for 2 h, cooled to room temperature and immediately used.
Visible spectrophotometric method
Determination of ammonium and thiocyanate
Ammonium and thiocyanate were extracted from the samples with distilled water. The ammonium was determined using the ninhydrin method as described by Fisher. The thiocyanate was determined using the method described by Kolthoff .As a standard in all analysis, a solution of ammonium thiocyanate was prepared and standardized as described by Kolthoff .
Determination of thiourea. The thiourea was extracted from the samples with 0.012 mol L-1 of NaOH and it was determined using an adaptation of the p-benzoquinone method described by Zaia .For all samples, the absorbance was read at 420 nm and acetate buffer (1.0 mol L-1, pH=3.0) was used.
Determination of guanidine thiocyanate. About 3.0 mg of guanidine thiocyanate per mg of KBr was used as standard in all determinations. The absorbance of NH deformation at 1656 cm-1 was used as characteristic band of guanidine thiocyanate. Different internal standards were used for different samples. For the samples of ammonium thiocyanate and ammonium thiocyanate plus river sand, sulfate (5.2 µg of K2SO4 per mg of KBr) was used as internal standard and it was measured the absorbance of the asymmetric stretch of sulfate (1111 cm-1). For the samples of ammonium thiocyanate plus salts of seawater, ammonium thiocyanate plus salts of seawater plus river sand, ammonium thiocyanate plus transition metals and ammonium thiocyanate plus transition metals plus river sand, iodate (27.9 µg of KIO3 per mg of KBr) was used as an internal standard and it was measured the absorbance of the stretch of IO (756 cm-1). For the samples of ammonium thiocyanate plus kaolin, boric acid (20.1 µg of H3BO3 per mg of KBr) was used as an internal standard and it was measured the absorbance of the BH2 deformation vibration (1194 cm-1). Depending on the concentration of guanidine thiocyanate in the samples, a different mass of the samples and the KBr (with appropriate internal standard) were weighted: from 20 to 100 mg for the samples and from 200 to 300 mg for the KBr. They were ground with an agate mortar and pestle until a homogenized mixture was obtained, after a disc pellet was prepared and the absorbances at wavelengths described above were measured. Raman spectra were obtained from solid samples. Because the samples are not homogeneous, several spectra were performed from different points of each sample used in the experiments.
Statistical analysis
Comparisons between means were assessed by using: Student’s t-test, ANOVA test and Student-Newman-Keuls test (S.N.K. test) at a significance level of p<0.05.
The analysis of thiourea (mg/100 g of NH4SCN) and guanidine thiocyanate (g/100 g of NH4SCN) in the samples of ammonium thiocyanate (A), ammonium thiocyanate plus river sand (B) and ammonium thiocyanate plus kaolin (C), heated for 3 days at different temperatures .The concentration of thiourea in the sample A. It should be also pointed out that the concentrations of thiourea in the samples B (150 ºC) and C (120 and 150 ºC) are very small when compared to the decomposition of ammonium thiocyanate in those samples This could mean that guanidine thiocyanate has been formed immediately after isomerization of ammonium thiocyanate to thiourea. Guanidine thiocyanate was not identified in the samples A and B heated at 80 ºC. Since, it is necessary to heat dry ammonium thiocyanate at obtained a yield of 89% for the synthesis of guanidine thiocyanate, however they did not use a condition of reaction that exist on the earth (temperature 300 ºC, equimolar amounts of lead salts and ammonium thiocyanate). Halmann, studying the condensation of glycine by cyanamide, reported that 12% of 0.05 mol L-1 cyanamide solution (pH 6.5) converted to cyanoguanidine after 5 days at 60 ºC. Lohrmann34 studied the formation of urea and guanidine by irradiation of ammonium cyanide. The best yields for guanidine formation using daylight radiation (iron, pH 8.4, 10 weeks) and 254 nm radiation (iron, chloride, pH 8.5, 7 days) were 20% and 8%, respectively. The yields for synthesis of guanidine obtained by Halmann33 and Lohrmann34 are not better than that showed in this paper, actually.
Conclusion
Guanidine is an important substance for the modern living beings as well as for prebiotic chemistry. Its cyclisation was very intrested using ammonium isothiocynates.The results show that river sand, kaolin, transition metals and salts of seawater catalyse the reaction between ammonium and thiocyanate in solid state under compatible prebiotic chemistry conditions. For all samples, it was obtained lower recoveries of thiocyanate and ammonium with an increasing of the temperature. At 120 ºC, only the recovery of thiocyanate (86.8%) in the sample with kaolin was statistically different (p<0.05) from the recovery of thiocyanate in the sample without kaolin. The recoveries of thiocyanate and ammonium in the samples with river sand or kaolin at 150 ºC were statistically different (p<0.05) from the recoveries of thiocyanate and ammonium in the sample without them. The decomposition of ammonium thiocyanate in the samples river sand or kaolin at 150 ºC was about 40%. The concentrations of thiourea or guanidine thiocyanate for all samples studied increased with an increasing of the temperature. The concentrations of thiourea in the samples with river sand or kaolin at 120 or 150 ºC were not statistically different (p>0.05) from the concentration of thiourea in the sample without them. Kaolin showed to have more effect on the synthesis guanidine than river sand. Because, the concentrations of guanidine in the samples with kaolin (120 and 150 ºC) were always bigger than in the samples with river sand (120 and 150 ºC). Salts of seawater did not show to increase the decomposition of ammonium thiocyanate even when river sand was added to the samples. However, the decomposition of ammonium thiocyanate in the samples with transition metals plus river sand was about 30%. The salts of seawater or transition metals plus ammonium thiocyanate plus river sand showed to increase the concentration of thiourea (seawater: transition metals guanidine (seawater: transition metals:) when they were compared to the samples of ammonium thiocyanate plus river sand. When the results obtained with sample of ammonium thiocyanate were compared to the samples of ammonium thiocyanate (at 120 ºC) plus salts of seawater or transition metals also an increased on the production of thiourea and guanidine, respectively. The yield for the synthesis of guanidine in this paper is better than that obtained by other authors.
Acknowledgement
The authors thanks to Dr. S.D. Kaushik (Principal, L.R. (P.G.) College, Sahihabad),) prof V.k Rastoge(meerut university), and finally We are greateful The Chemistry Department of Delhi University for their spectral help.
References
- R.E. Dickerson, J. Mol, Biol, 57, 1 (1971).
- Ojima, I.; Charkravarty S.; Dong O, Biorg Med Chem, 3, 337-360 (1995).
- Kovacevic, B. Maksic Z, B. Org Lett. 3, 1523, (2001).
- S.M. Mohamed, Ind J. of Chemistry 45B ,1453, ( 2002)
- Sudhir kumar,upma singh,lakshman singh;,orient.j.of chem.,vol.24(1),223,(2008)
- Kienzle, F.: Kaiser; Madhukar, Eur Med Chem, 17, 547 (1982).
- Katritzky, A.R. Cai, X, Rogovoy, B, J, Comb Chem, 5, 392 (2003).
- Summary of guanidylation Powell D.A. J. Org Chem. 68, 2300 (2003).
- Lindstrom, P. Wierney J, Tetrahedron 57, 9225 (2001).
- Mikolayczyk M. : Kulbasinski, Tetrahedron 37, 233 (1981).
- Bretherick S, Handbook of Reactive Chem. Hazards 5th ed. P4294, 1995.
- Matsumura N.: Konishui T. J. Heteorycl Chem. 39, 189 (2002).
- Ferris A.F. J. Org. Chem. 71b, 28 (1963).
- Y.J. Liu, H. : Dick J. Am. Chem. Soc. 124, 19591 (2002).
- Ishikaa, F. : Kosayana, A. : Nakamura Chem. Pharma Bull 26, 3657 (1978).
- sudhir kumar;,lakshman singh and upma singh;,asian .j.of chem, 20(6),4957.;(2008)
- Halamann M,Arc Biochem.Biophy.128,808,(1968)
- Lohrmann R:,J Mol Evol. 1,263,(1972)
- Dowler M, J, Ingmanson,D .E;, Nature.91,279,(1979)
- Summers, D,P. :,Origin Life Evol Biosph ;29,33,(1999)

This work is licensed under a Creative Commons Attribution 4.0 International License.









