Mechanistic Study of Chromium (VI) Catalyzed Oxidation of Benzyl Alcohol by Polymer Supported Chromic Acid
Vilas Y. Sonawane
Department of Chemistry, B. Raghunath Arts, Science and Commerce, College, Parbhani, 431 401 (India).
The oxidation of benzyl alcohol has been studied spectrophotometrically to compare the polymer substrate supported reagent with a commercially available cross-linked polymeric reagent. The reagent supported on anion exchange resin was found to be more efficient in the oxidation reaction. The reagent is very easily separated from the reaction mixture and can be manually removed from the reaction mixture, which remains clear during and after the reaction. The kinetic of oxidation of benzyl alcohol with chromic acid supported on anion exchange resin like Tulsion-T-52 A [Cl-] in 1, 4-dioxane has been studied. The reaction is found to be of zero order each in concentration of alcohol and oxidant. The oxidation product have been isolated and characterized by its derivative, UV and FT-IR spectral studies. The effect of substituent’s on the rate of oxidation and the thermodynamic parameters were determined with respect to slow step of mechanism.
KEYWORDS:Mechanistic; Catalyzed Polymer- supported chromic acid; Oxidation; Benzyl alcohol; Tulsion-T-52 A [Cl-]
Download this article as:| Copy the following to cite this article: Sonawane V. Y. Mechanistic Study of Chromium (VI) Catalyzed Oxidation of Benzyl Alcohol by Polymer Supported Chromic Acid. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Sonawane V. Y. Mechanistic Study of Chromium (VI) Catalyzed Oxidation of Benzyl Alcohol by Polymer Supported Chromic Acid. Orient J Chem 2011;27(1).Available from: http://www.orientjchem.org/?p=24790 |
Introduction
Chromium trioxide dissolves in water to form Chromic acid. Chromic acid being one of the most versatile available oxidizing agents. Now a day the development of newer chromium (VI) reagents 1-6 for the oxidation of organic substrates continues to be of interest. Chromium is one of the most widely distributed heavy metals in the earth’s crust. It is normally found into oxidation states i.e. Cr (III) and Cr (V). Chromium is required in small quantities as an essential trace metal. Most of the biological tissues contain Cr (III) which is usually non toxic, where as Cr (VI) is a highly toxic for the metal to the organism.7
In the present investigation now herewith report the oxidation of benzyl alcohol by polymer- supported chromic acid. Tulsion-T-52 A [Cl–] is the strong anion exchange resin are supported on chromium (VI) oxide and used as an oxidant.
Experimental
Preparation of supported oxidizing agent
The supported oxidizing agent was prepared by reported method.8-10 The chloride form of Tulsion-T-52 A [a macro reticular anion exchange resin] containing a quaternary ammonium group [10 x 10-3 kg] was stirred with a saturated solution of chromium trioxide [5 x 10-3 dm3] in water [30 x10-3 dm3] for 30 minutes at room temperature using a magnetic stirrer. The chloride ion was readily displaced and HCrO4– form of resin was obtained in 30 min. The resin was successively rinsed with water, acetone and THF and finally dried in vaccum at 323 K for 5h. The dried form of the resin was stored and used throughout the kinetic study.
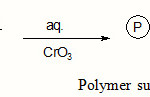 |
Scheme 1 Click here to View scheme |
Determination of the capacity of chromate form of the polymeric reagent
The capacity of the chromate form of Tulsion-T-52 A [Cl–] polymeric reagent was determined by iodometrically. The capacity of the chromate form of resin was 6.90 mmol/g and used for kinetic study throughout work. The loading was also determined by elemental nitrogen analysis and was found to be 6.82 mmol/g.
Chemicals and Reagents
All reagents used were of Analytical Grade and all solutions were prepared with double distilled water.
Method of kinetics of oxidation procedure
The reaction mixture for the kinetic run was prepared by mixing benzyl alcohol, oxidant and solvent. The reaction was carried out either constant stirring using magnetic stirrer and at a constant temperature 318 ±1 K. At different time interval, the reaction mixture was withdrawn using a micropipette. The aliquot thus withdrawn was taken in a stoppered test tube containing 5 x 10-3 dm3 of 1, 4-dioxane and subjected to spectral analysis. The absorbance of the product formed was measured using SL 159 UV-visible spectrophotometer. Duplicate kinetic runs showed that the rate constants were reproducible to within ±3 %.
Induced polymerization test
Mixing of oxidant, benzyl alcohol and solvent at 318 K with continuous stirring initiation of reaction. After 30 min, the reaction mixture was withdrawn in a test tube and acrylonitrile was added. The mixture after dilution with distilled water formed a copious precipitate. The precipitate formed, due to polymerization of acrylonitrile, indicates formation of a free radical species in the reaction 11. It was also confirmed by ESR spectral analysis as well as on diluting the reaction mixture with acidified methanol, a precipitate formed, suggested the possibility of free radical interventation in the reaction.
Product analysis
The oxidation of benzyl alcohol leads to the formation of benzaldehyde. The product formed was analyzed by their 2, 4-dinitrophenylhydrazine derivatives. The precipitated 2, 4-DNP was filtered off, the product is then vacuum dried, weighed and recrystallised from ethanol. The yield of 2, 4-DNP recrystallisation with the DNP of benzaldehyde was 99%. The product benzaldehyde also identified either by comparison with authentic sample or by UV, FT-IR spectral and elemental analysis. The IR spectra was recorded on a Jasco FT-IR spectrophotometer using KBr pellets. The melting point of 2,4-DNP derivative of benzaldehyde is 509K.
UV spectrum λmax 247 nm.
IR data: – A sharp band at 1690 cm-1 for -CHO stretching mode, 1582 cm-1 aromatic
(- C = C-), 3060 cm-1 (-C- H stretch).
Results and Discussion
Effect of oxidant
The order with respect to weights oxidant is zero, as the plots of absorbance against time were linear in all runs and observed rate constant are fairly constant between 120 to 180 x 10-6 kg of oxidant at constant concentration of solvent (1, 4- dioxane, 10 x 10-3 dm3) and benzyl alcohol (15 x 10-3 mol/dm-3), the effect of varying weights of oxidant on zero order rate constant as shown in Table-1.
Table 1: Effect of oxidant on reaction rate
| Oxidant x 10– 6 kg | 120 | 140 | 160 | 180 |
| k x 10– 4 min-1 | 2.10 | 2.15 | 2.25 | 2.30 |
Effect of concentrations of benzyl alcohol
At a varying concentration of benzyl alcohol [10 to 25 x 10-3mol/dm3], constant weights of oxidant [140 x 10-6 kg] and constant concentration of solvent [1,4-dioxane, 10 x 10-3dm3], zero order rate constant [Table- 2] was found.
Table 2: Effect of concentrations of benzyl alcohol on the reaction rate
| Benzyl alcohol | 10 x 10-3mol /dm3 | 15x 10-3mol /dm3 | 20 x 10-3mol /dm3 | 25 x 10-3mol /dm3 |
| k x 10– 4 min -1 | 3.20 | 4.10 | 4.50 | 5.10 |
Effect of dielectric permittivity
It was found that as the dielectric constant of the medium increased, this including r* < r [Where r* and r refer to the radii of the reactant species and activated complex respectively] at constant concentration of benzyl alcohol [15 x 10-3 mol/dm3] and constant concentration of oxidant [140 x 10– 6 Kg], solvent [10x 10-3dm3] as shown in Table-3.
Table 3: Effect of dielectric permittivity of the medium on the reaction rate
| Solvent[10×10-3dm3] | C6H12 | CCl4 | 1,4-dioxane | CHCl3 |
| Dielectric constant | 2.00 | 2.17 | 2.28 | 4.81 |
| k x 10– 4 min -1 | 2.20 | 3.10 | 5.10 | 5.35 |
Effect of temperature
The reaction was carried out at four different temperatures under conditions to study the effect of temperatures on the rate of reaction. It was observed that, the rate of reaction increased with an increase in the temperature. [Table-4]. The thermodynamic parameters like energy of activation [Ea], enthalpy of activation [∆H#], entropy of activation [∆S#] free energy of activation [∆G#] and frequency factor [A] were calculated by determining values of k at different temperatures. [Table-5].
Table 4: Effect of temperature on the reaction rate
| Temperature K → | 313 | 318 | 323 | 328 |
| k x 10– 4 min -1 | 3.20 | 4.30 | 4.90 | 6.20 |
Table 5: Thermodynamic parameters for the oxidation of benzyl alcohol
| Energy of activation [Ea] KJ mol-1 | 84± 4 |
| Enthalpy of activation [∆H#] KJ mol-1 | 49± 3 |
| Entropy of activation [∆S#] JK mol-1 | -55± 2 |
| Free energy of activation [∆G#] KJ mol-1 | 256± 2 |
| Frequency factor [A] X 10-5 s-1 | 3± 0.5 |
Discussion
Several sets of experiments with various weights of oxidant, concentration of benzyl alcohol and change in solvent were carried out. The reaction was found to be zero order. The proposed path for the reaction of chromium (IV) then makes possible a different mechanism for oxidation of benzyl alcohol. According to Westheimer and Watanable [12], subsequent steps must involve chromium (IV) as shown in Scheme (II) and (III).
Cr IV + Cr VI →2CrV (1)
CrV + R2CHOH → R2 C = O + CrIII + 2H+ (2)
2Cr V → CrIII + Cr V (3)
Cr V +Red → Cr III + oxi (4)
Westheimer then proposed that the oxidation of primary alcohols proceeded via acid chromate ester intermediates.
R2CHOH + HCrO4 + H+ →R2CHOCrO3H + H2O (5)
R2CHOCrO3H + H+ →R2CHOCrO3H2+ (6)
If the oxidant supported on polymer, which has certain advantages over homogeneous
reaction, the intermediate chromium (IV) will further oxidize another molecule of
benzyl alcohol to form a free radical species. Thus based on experimental results, obtained for the oxidation of benzyl alcohol by polymer support, the reaction was found to be 0th order. Initially Cr (VI) is reduced to Cr (IV). It is likely to react with another Cr (VI) to generate Cr (V) which is then reduced in a fast step to the ultimate product Cr (III). Such a sequence of reactions in Cr (VI) oxidation is well known.13-15 The mechanism is suggested in Scheme (IV) and involves ester formation.
The polymer supported reagent reacts with a molecule of benzyl alcohol to form a chromate ester.
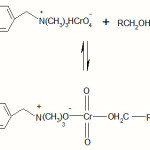 |
Scheme 2 Click here to View Scheme |
The ester formed will decompose into aldehyde and the intermediate chromium (IV) will be formed in the second and slow step.
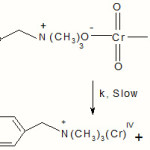 |
Scheme 3 Click here to View scheme |
The intermediate chromium (IV) thus reacts with another benzyl alcohol molecule to produce a free radical species.
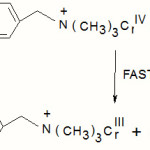 |
Scheme 4 Click here to View scheme |
Subsequently the free radical reacts with another oxidant site in the polymeric reagent in a fast step leading to the formation of chromium (V).
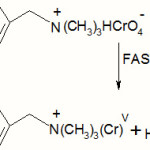 |
Scheme 5 Click here to View Scheme |
R = C6H5
Scheme IV
The intermediate chromium (V) in the last step reacts with benzyl alcohol produce benzaldehyde.
Acknowledgements
I wish to express my deep sense of gratitude to Hon’ble Adv.Ashok Soni, President, Godavari Shikshan Prasarak Mandal’s, B. Raghunath Arts, Science and Commerce,College Parbhani and Prof. Smt. Nandini P. Hilage, Department of Chemistry, Shivaji University, Kolhapur.
Conclusion
According to Scheme IV, a second order rate law is expected. But since the first step of ester formation occurs in solid phase and assuming that this equilibrium does not contribute to the rate of reaction. We obtained zero order dependence with rate constant k of the second slow step in which product benzaldehyde was obtained. Based on the experimental observations a probable mechanism is suggested.
References
- E. J. Corey and G. Schmidt, Tetrahedron Lett., 20, 399 (1979).
- M. N. Bhattacharjee, M.K.Choudhari,H. S.Dasgupta,N.Roy and D. T. Khating, Synthesis., 58 (1982).
- E. J. Corey, E.P.M.Barette and P.A. Margrious, Tetrahedron Lett., 24, 5855 (1985).
- F. Climinale, M.Camporeale, R.Mello, L.Troisi and R.Curci , J. Chem.Soc.,Perkon Trans., 2, 417 (1989).
- G. G. Sharma and M. K. Mahanti , Bull. Soc. Chem. Fr., 128, 449 (1991).
- K. Balasubramanian and V. Pratibha, Indian J. Chem., Sec. B., 25, 326 (1986).
- B. Narayana and Tam Cherian , J. Braz. Chem. Soc., 16, 197 (2005).
- A.L.Jawanjal and N.P.Hilage, Indian J.Chem.,44A, 1827 (2005). S.A. .Kakde and N.P.Hilage, Trans. Metal Chem., 32, 940-943 (2007).
- G. Cainelli, G. Cardillio, M. Orena and S. Sardri, J. Am. Chem. Soc., 98, 6767 (1976).
- T. Brunlet, C. Jouitteau and G. Gelhard, J. Org. Chem., 51 , 4016 (1986 ).
- W. A. Mosher, H. Clement and R. L. Hillard , J. Am .Chem. Soci., 29, 565 (1993).
- W. Watanabe and F. H. Westheimer, J. Chem . Phys., 61, 17 (1979).
- M. M. Salunke, D. G. Salunke, A. S. Kanade, R. B. Mane and P. P. Wadgaonkar , Synth Commun.,2B, 1143 (1990).
- J. Matsuo, A. Kawana, K. Pudhon and T. Mukaiyama, Chem. Lett., 250 (2002).
- R. O. Hutchins, N. R. Natale and W. J. Cook, Tetrahedron Lett., 4167 (1977).

This work is licensed under a Creative Commons Attribution 4.0 International License.









