Synthesis and Characterisation of Polystyrene Supported Chelating Ligands and their Transition and Inner Transition Metal Complexes
Jagat Pal Gangwar and P.N. Saxena
Department of Chemistry, Bareilly College, Bareilly - 243 001 (India).
New polystyrene supported coordination compounds have been synthesized by the reaction of metal salt / metal coordination compound with the polystyrene supported base (PS-LH2)(obtained by the reaction of chloromethylated polystyrene, 3-formylsalicylic acid and ethanolamine). The compounds have the formulae:- PS-TiCl.2DMF, PS-VCl.2DMF, PS-MnCl. 2DMF,PS-FeCl .2DMF,PS-RuCl.2DMF,PS-Ru.3DMF,PS-MoO2.DMF,PS-MoOCl.DMF,PS-UO2.DMF and PS-CoCl.2DMF (DMF=dimethylformamide). The compounds have been characterized by elemental analyses, IR, electronic and magnetic susceptibility measurements. The Ti(III), V(III), Mn(III), Fe(III), Ru(II) and Mo(V) compounds are paramagnetic while Mo(VI) and U(VI) compounds are diamagnetic. The shifts of the (C=N) (azomethine), (C-O) (phenolic) and (C-O) (alcoholic) stretches have been monitored to find out the donor sites of PS-LH2. An octahedral structures for all the compounds have been suggested for the compounds.
KEYWORDS:Polystyrene; Chelating Ligands; Transition metal and complexes
Download this article as:| Copy the following to cite this article: Gangwar J. P, Saxena P. N. Synthesis and Characterisation of Polystyrene Supported Chelating Ligands and their Transition and Inner Transition Metal Complexes. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Gangwar J. P, Saxena P. N. Synthesis and Characterisation of Polystyrene Supported Chelating Ligands and their Transition and Inner Transition Metal Complexes. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=24766 |
Introduction
The reaction of polymer-anchored ligand with metal ions provides an easy route for the synthesis of immobilized transition metal compounds. Several polymer-anchored ligands like porphyrins, polydentateamines, crown ethers, iminodiautic acid, acetyacetone, tripeptide and their metal absorbing property have been reported (1,2). However, some of these studies are limited by leaching of the metal ions from the polymer and no concerted effort has been given to study the structural aspects of the metal bound to the chelating resins. A structural study of such metal bound polymers seems useful and interesting in view of their numerous applications(3-5). Such as in organic synthesis, immobilization of enzymes, biological systems, application of dyes, water treatment, chromatography, catalysis, as substrate carriers, protecting groups and metal absorbing agents. The Schiff bases are the most versatile and thoroughly studied ligands in coordination chemistry. On account of their pronounced coordinating properties, a number of Schiff bases have been anchored on polystyrene matrix (6-10). In this paper, we report the syntheses and characterization of coordination compounds of the polystyrene supported Schiff base.
Materials and Methods
All the chemicals and reagents used were of AR grade or equivalent purity. Titanium chloride was prepared in the lab by standard method given in chemical literature.
Chloromethylated polystyrene (PS-Cl)(containing 1.17m mol Cl per g of resin and 2% crosslinked with divinylbenzene) [Fluka A G (Switzerland)]. The metals used were Ti(III)chloride, vanadium(III) chloride, Manganese(III)chloride, Iron(III) chloride, Cobalt(III) chloride, Ruthenium(III) Chloride, Ruthenium(II) chloride, Oxomolybdenum(V) chloride, Dioxomolybdenum(VI), Dioxouranium(VI). The solvents used were methanol, acetone, ethylacetate, triethyl amine and DMF and 3-formylsalicylic acid.
Synthesis of Polystyrene-3-formylsalicylate(PS-Fsal):-
Chloromethylated polystyrene (PS-Cl)(1.0g) was swelled in 20 ml of DMF for about 50min. A solution of 3-formylsalicylic acid (0.789,4.68 m mol) in 20 ml of DMF was added to this solution. 100ml of ethyl acetate and 1.5g (15m mol) of triethylamine were added to above reaction mixture. This mixture was then heated under reflux for 8h. with magnetic stirring. The mixture was then allowed to cool to room temperature. The pale yellow resin was suction filtered, washed with DMF, ethyl acetate, ethanol and acetone and was dried in vacuo at room temperature.
Synthesis of polystyrene anchored Schiff base derived from 3- formylsalicylic acid and ethanolamine
It was carried out in two steps by reported method(II).
a) In the first step, PS-Cl and 3-formylsalicylic acid reacts together to form polystyrene 3-formylsalicylate.
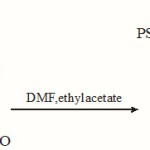 |
Scheme 1 Click here to View scheme |
b) In the second step, PS-Fsal reacted with ethanolamine in 1:4 molar ratio to form PS-LH2(I).
Polystyrene-3-formylsalicylate(PS-Fsal) (1.0g) was swelled in 50ml of DMF for 45min. To this suspension, a 50 ml. DMF solution 0.286 g (4.68 mmol) and 100 ml ethyl acetate were added and mixture was stirred magnetically. It was refluxed for 8h and then cooled to room temperature. The yellow coloured polystyrene anchored ligand was suction filtered, washed with DMF, ethyl acetate methanol and acetone. It was then dried in vacuo at room temperature.
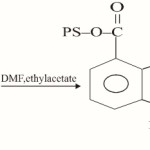 |
Scheme 2 Click here to View scheme |
Characterisation of Ligands
The ligands were characterised by the determination of melting points, elemental analyses and recording of infrared spectra.
General Method for the synthesis of PS-LM. x DMF
(where M = metal, x = no. of DMF molecules)
The polystyrene-anchored Schiff base(PS-LH2) (0.5.g) was suspended in DMF (20.ml) for 45 min. To this suspension, a DMF solution of appropriate metal salt was added. The mixture was refluxed for 8h with magnetic stirring. It was then allowed to cool to room temperature. the product was suction filtered, washed with DMF, ethanol, methanol and acetone and then dried in vacuo at room temperature.
PS–LH2 + MCl3 ![]() PS–MCl.2DMF + 2HCl ( M = M (III )
PS–MCl.2DMF + 2HCl ( M = M (III )
The metal contents and coordinated DMF in the polystyrene supported coordination compounds were analysed as per the reported method (9). The magnetic susceptibilities were determined by the Gouy method using Hg[Co(NCS)4]as the calibrant. A double ended one side sealed tube with zero diamagnetic susceptibility was used for measuring the magnetic susceptibility. The diamagnetic correction of the metal-ligand system was calculated using the Pascal’s constant (9) and following a procedure specially designed for polystyrene anchored compounds (9). Electronic spectra were recorded on a Beckman DU spectrophotometer attached with a reflectance arrangement. Infrared spectra were recorded in KBr pellets in the range 400-4000cm-1 on a Nicolet FTIR spectrophotometer using polystyrene as a calibrant.
Results and Discussion
The synthesis of polystyrene-anchored Schiff base(I) derived from 3-formylsalicylic acid and ethanolamine was carried out in two steps. In first step, PS-Cl and 3-formylsalicylic acid reacts together to form polystyrene 3-formylsalicylate (PS-Fsal). In second step, PS-Fsal reacts with ethanolamine in 1:4 molar ratio to form PS-LH2.
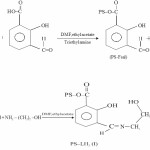 |
Scheme 3 Click here to View scheme |
The IR spectra shows (C=O) (carboxylic) stretch in 3-formylsalicylic acid occurs at 1660 cm-1. The appearance of a new band at 1730cm-1 due to (C=O) (ester) in PS-Fsal confirms the covalent bond formation via ester linkage between 3-formylsalicylic acid and PS-Cl (7). The (C=N) (azomethine), (C-O) (phenolic) and (C-O) (alcoholic) stretches occurs at 1625,1520 and 1210 cm-1 respectively in the Schiff base derived form salicylaldehyde and ethanolamine(13). These band occur at 1630,1515 and 1225 cm-1 respectively in PS-LH2.
The analytical data has suggested 1:1 metal ligand ratio for the complexes. All the complexes are non electrolytes. The complexes of Ru(II), UO2, MoO2 are diamagnetic in nature as expected for d0 and f0 configuration. All other complexes are paramagnetic in nature and the values of magnetic moment are given in the table.
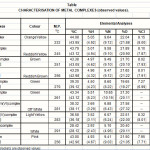 |
Table 1 Click here to View table |
Electronic Spectra
The electronic spectrum of titanium(III) complex has shown a single broad band at 19500 cm-1 which has been assigned to 2T2g→2Eg for octahedral symmetry (4).
The electronic spectrum of vanadium(III)complex showed band at 16000cm-1 with a shoulder at 21000cm-1. The low energy band has been assigned to 3T1g→3T2g where as the high energy band to 3T1g→3T1g(p) transitions respectively. These are characteristic of octahedral geometry (8).
In the electronic spectrum of manganese(III) complex exhibits two bands at 19000 and 13000cm-1 assignable to 5B1→5B2 and 5B1→5Eg transitions respectively(10) and are characteristic of octahedral geometry(10).
The electronic spectrum of iron(III) complex exhibits three bands at 12730,19650 and 25000cm-1 assignable to 6A1g→4T1g, 6A1g→4T2g and 6A1g→4A1g transitions respectively and characteristic of octahedral symmetry(II).
The electronic spectrum of ruthenium(III)complex displays bands at 13650cm-1, 17500cm-1 and 22550cm-1 which may be assigned to 2T2g→4T1g,2T2g→4T2g and 2T2g→2A1g,2T1g transitions respectively(12). These are characteristic of Ru(III) octahedral complexes (12).
The electronic spectrum of ruthenium(II) complex showed a single band at 23000cm-1. This band has been assigned to the charge transfer transition arising from the excitation of an electron from metal t2g level to the unfilled molecular orbital derived from p* level of the ligand in accordance with the assignments made for other similar octahedral Ru(II) complexes (13).
The electronic spectrum of dioxomolybdenum(VI) complex shows absorption bands of considerably high intensity at 35000 & 30000 cm-1. These bands may be assigned to ligand metal charge transfer transitions (14).
The electronic spectrum of oxomolybdenum(V) complex suggested octahedral geometry with a strong tetragonal distortion resulting from molybdenum-oxygen bond. The spectrum exhibits three absorption bands at 13500,19600 and 25000cm-1 assignable to the transitions 2B2→2E(dxy→dxz,dyz), 2B2→2B1(dxy→dx2-y2) and 2B22A1(dxydz2) transitions respectively(14).
The electronic spectrum of dioxouranium(VI) complex shows bands at 21750, 25200 and 37000cm-1 consistant with vibroanic structure of triatomic entity of UO2 group(16). This complex has octahedral geometry(16).
The electronic spectrum of the cobalt(III) complex displays bands at 15200,21100 and 23400cm-1 which may be assigned to 1A1g→3T2g,1A1g→1T1g and 1A1g→1T2g transitions respectively. These are similar to those reported for other six co-ordinated Co(III) complexes(18).
I.R. Spectra
In the polystyrene anchored compounds, the(C=N) (azomethine) undergoes a negative shift by 5-30cm-1, n(C-O) (phenolic) undergoes a positive shift by 10cm-1 and n (C-O) (alcoholic) respectively. In the polystyrene anchored Ti(III) complex of this ligand, the n (C=N) undergoes a negative shift 15 cm-1 n (C-O)(phenolic) undergoes a positive shift by 10cm-1 and n (C-O) (alcoholic) undorgoes a negative shift by 5-30cm-1. These shifts in IR frequencies after co-ordination with metal ions indicate the ONO donor tridentate behaviour of the ligand (13) of PS-LH2. The IR data rule out the presence of a dimetallic structure and indicate a monometallic structure as in the case of a dimetallic structure, the n (C-O)(phenolic) stretch is expected to undergo a positive shift(14) by > 10cm-1. DMF shows a band at 1680cm-1 due to n (C=O) stretch(8). This band has shifts to lower energy by 5-30cm-1 in the compounds indicating oxygen co-ordination of DMF(8).PS-LUO2.DMF shows the nasy(O=U=O) at 905cm-1 and this band occurs in the usual range (870-950cm-1) observed for majority of trans-UO2 compounds (9,14).
Conclusion
On the basis of above mentioned studies, octahedral geometry have been proposed for all the complexes. In case of oxovanadium(V) a distortion is expected due to Mo=O moiety.
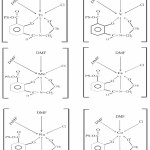 |
Scheme 4 Click here to View scheme |
Acknowledgement
The authors are thankful to Dr. R.P. Singh, Principal, Bareilly College and
Dr. R.B. Singh, Head, Dept. of chemistry for providing facilities to carry out this research work.
References
- Topich J, Inorg chem Acta, vol.46, pp-197(1980).
- El-Nassar AA & Parish RV, J chem soc, Dalton Trans Vol.19 , pp 3463(1999).
- Suzuki TM, Yokoyama T. Matsunga H & Kimura J. Bull chem soc, Japan 59(1986)865.
- Hutchins MS & Chapman TK, Tetrahedron Lett, 35(1994)4055.
- Vaino RA & Janda KD, J comb chem, 2(2000)579.
- Drago RS, Goul J, Zombeck A & Staub DK, J Am chem soc, 102(1980) 1033; Matsushita T, Sakiyama J, Fujiwara M & Shono T, chem Lett (1980)1577; Topich J, Inorg chem, 21(1982) 2079, Suzuki TM & Yokoyama T. Polyhedran, 2(1983) 127.
- Syamal A & Singh MM, J. Polym Mater, 6(1989)175; 9(1992)105.
- Syamal A & Singh MM, India J. chem, 31A(1992)110;32A(1993)42.
- Syamal A, Singh MM, & Kumar D, React Funct Polym, 21(1993)45, 140.
- Syamal A, Singh MM, & Kumar D, React Funct Polym, 39(1999)27.
- Topich J Inorg. chem Acta, 46(1980)197.
- Dutta RL & Syamal A, Elements of magnetechemistry, 2nd Edn ( Affiliated East West Press Pvt Ltd, New Delhi), (1993)7.
- Syamal A & Kumar D, J Less- Common Metals, 71(1980)113.
- Alyea FC, Malek A & Kazi A K, Trans met chem, 6(1981)223.

This work is licensed under a Creative Commons Attribution 4.0 International License.









