Synthesis and Screening of Anti-inflammatory Activity of Benzofuran Derivatives Bearing Oxadiazole
Balasaheb Y. Mane1* and S. Vidyadhara2
¹PDVVPF’s College of Pharmacy, Vilad Ghat, MIDC, Ahmednagar - 414 111 (India).
²Chebrolu Hannumaihha Institute of Pharmaceutical Sciences, Guntur, Andhra Pradesh (India).
A series of novel benzofuran analogs of fenamates were synthesized, where carboxylic acid functionality was modifieds to five membered heterocyclic ring. The structures of newly synthesized compounds were established by the 1H-NMR, IR and elemental analyses data. All the new compounds were screened for anti-inflammatory activity by carrageenan induced rat paw edema method. Some of the compounds were found to possess good anti-inflammatory activity. Diclofenac sodium was used as standard.
KEYWORDS:Benzofuran; Fenamates; Anti-inflammatory activity; Oxadiazole
Download this article as:| Copy the following to cite this article: Mane B. Y, Vidyadhara S. Synthesis and Screening of Anti-inflammatory Activity of Benzofuran Derivatives Bearing Oxadiazole. Orient J Chem 2011;27(3). |
| Copy the following to cite this URL: Mane B. Y, Vidyadhara S. Synthesis and Screening of Anti-inflammatory Activity of Benzofuran Derivatives Bearing Oxadiazole. Orient J Chem 2011;27(3). Available from: http://www.orientjchem.org/?p=24635 |
Introduction
The fenamates are the class of NSAID that have common structural features of an N-arylanthranilic acid such as mefenamic acid, meclofenamic acid and flufenamic acid.1-3 These are the first line therapeutic agents for the clinical treatment of various inflammatory disorders.4 In view of this several anthranilic acid derivatives have been prepared for investigation of anti-inflammatory activity. Many such compounds were found to possess potent anti-inflammatory,5-7 cardiovascular8 and analgesic9 activities. Anthranilic acid derivatives are known to act by blocking the metabolism of anthranilic acid by the enzyme cyclooxygenase (COX) and thereby the production of prostaglandins. Inhibiting COX may also increases the conversion of arachidonic acid to proinflammatory leukotrienes via the enzyme 5-Lipoxygenase (5-LOX).10 Literature survey shows that modification of carboxylic group in anthranilic acid markedly modulates the activity. The replacement of the carboxylic acid functionality with five membered heterocyclic ring moieties not only retained the COX inhibitory activity of the parent but also inhibited 5-LOX.11 Thus these compounds are the balanced dual inhibitors and displays potent activity as anti-inflammatory agents. Among the wide variety of five membered heterocycles that have been explored for developing pharmaceutically important molecules 1, 3, 4-oxadiazole derivatives have played a vital role in the medicinal chemistry. There are large numbers of synthetic compounds with oxadiazole nucleus used for antibacterial12-16, antifungal17-20, analgesic and anti-inflammatory21-24 activities when properly substituted in 2 and 5 positions. Various biological applications have been reported for benzofuran derivatives, such as antimicrobial, anti-inflammatory and analgesic activities25-28. The broad spectrum of therapeutic values of benzofuran prompted us to synthesize benzofuran analogs of anthranilic acid derivatives in which benzene moiety of anthranilic acid is replaced by benzofuran moiety.
Experimental
General procedure
Melting points were determined in open capillary tubes and are uncorrected. The FT-IR spectra were recorded in KBr on SHIMADZU FTIR-8400S spectrophotometer. 1HNMR spectra were recorded in CDCI3 on a Varian Mercury YH-300, using TMS as an internal standard.
Typical procedure for the synthesis of 5-(3-Arylaminobenzofuran-2-yl)-1, 3, 4-oxadiazole-2-thiol: (2a-h)
To a cold stirred solution of (1a-h) (0.001 mol) in ethanol (50 ml) containing potassium hydroxide (0.01 mol), carbon disulphide (0.05 mol) was added gradually. The reaction mixture was heated under reflux on a steam bath until hydrogen sulphide evolution ceased. Ethanol was removed by distillation under reduced pressure and residue was stirred with water, filtered and filtrate was neutralized with dilute hydrochloric acid. The product was filtered, washed with water and recrystallised from ethanol to get compounds (2a-h). The physical, elemental and spectral analysis data of the compounds (2a–h) are as follows.
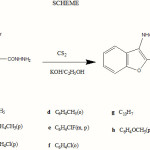 |
Scheme 1 Click here to View scheme |
5-(3-(phenylamino) benzofuran-2-yl)-1, 3, 4-oxadiazole-2-thiol (2a):
Yield: 87%; m.p. 225-226°C. Anal. Calcd. For C14H11N3O2S: C, 58.94; H, 3.85; N, 14.73%. Found: C, 58.90; H, 3.85; N, 14.70%. IR (KBr, cm−1): 3360 (N-H), 1610 (C-N), 1182 (C=S). 1H-NMR (400 MHz, CDCl3, δ / ppm): δ 7-7.8 (m, 9H, ArH), δ 9.08 (s, 1H, NH), δ 10.51 (s, 1H, SH).
5-(3-(p-tolylamino) benzofuran-2-yl)-1, 3, 4-oxadiazole-2-thiol (2b):
Yield: 85%; m.p. 210-211°C. Anal. Calcd. For C17H13N3O2S: C, 63.10; H, 3.95; N, 12.95%. Found: C, 63.15; H, 4.02; N, 13.00%. IR (KBr, cm−1): 3350 (N-H), 1500 (C-N), 1190 (C=S). 1H-NMR (400 MHz, CDCl3, δ / ppm): δ 2.5 (s, 3H, ArCH3), δ 7-7.7 (m, 8H, ArH), δ 9.10 (s, 1H, NH), δ 10.51 (s, 1H, SH).
5-(3-(4-chlorophenylamino) benzofuran-2-yl)-1, 3, 4-oxadiazole-2-thiol (2c):
Yield: 80%; m.p. 205-206°C. Anal. Calcd. For C16H10N3O2SCl: C, 55.90; H, 2.85; N, 12.20%. Found: C, 55.89; H, 2.91; N, 12.22%. IR (KBr, cm−1): 3355 (N-H), 1490 (C-N), 1190 (C=S). 1H-NMR (400 MHz, CDCl3, δ / ppm): δ 2.5 (s, 3H, ArCH3), δ 7-7.7 (m, 8H, ArH), δ 9.10 (s, 1H, NH), δ 10.51 (s, 1H, SH).
5-(3-(o-tolylamino) benzofuran-2-yl)-1, 3, 4-oxadiazole-2-thiol (2d):
Yield: 85%; m.p. 200-201°C. Anal. Calcd. For C17H13N3O2S: C, 63.10; H, 3.95; N, 12.95%. Found: C, 63.15; H, 4.02; N, 13.00%. IR (KBr, cm−1): 3350 (N-H), 1495 (C-N), 1185 (C=S). 1H-NMR (400 MHz, CDCl3, δ 2.4 (s, 3H, ArCH3), δ 7-7.8 (m, 8H, ArH), δ 9.09 (s, 1H, NH), δ 10.55 (s, 1H, SH).
5-(3-(3-chloro-4-fluorophenylamino) benzofuran-2-yl)-1, 3, 4-oxadiazole-2-thiol (2e):
Yield: 79%; m.p. 180-182°C. Anal. Calcd. For C16H9N3O2SClF: C, 53.10; H, 2.45; N, 11.60%. Found: C, 53.11; H, 2.48; N, 11.61%. IR (KBr, cm−1): 3360 (N-H), 1492 (C-N), 1185 (C=S). 1H-NMR (400 MHz, CDCl3, δ 7.5-8 (m, 7H, ArH), δ 9.08 (s, 1H, NH), δ 10.52 (s, 1H, SH).
5-(3-(2-chlorophenylamino) benzofuran-2-yl)-1, 3, 4-oxadiazole-2-thiol (2f):
Yield: 82%; m.p. 185-186°C. Anal. Calcd. For C16H10N3O2SCl: C, 55.90; H, 2.85; N, 12.20%. Found: C, 55.89; H, 2.91; N, 12.22%. IR (KBr, cm−1): 3360 (N-H), 1490 (C-N), 1185 (C=S). 1H-NMR (400 MHz, CDCl3, δ 7.2-7.9 (m, 8H, ArH), δ 9.10 (s, 1H, NH), δ 10.50 (s, 1H, SH).
5-(3-(naphthalene-1-ylamino) benzofuran-2-yl)-1,3,4-oxadiazole-2-thiol (2g):
Yield: 75%; m.p. 189-190°C. Anal. Calcd. For C20H13N3O2S: C, 66.85; H, 3.60; N, 11.60%. Found: C, 66.85; H, 3.62; N, 11.69%. IR (KBr, cm−1): 3360 (N-H), 1490 (C-N), 1190 (C=S). 1H-NMR (400 MHz, CDCl3, δ 7-7.9 (m, 11H, ArH), δ 9.20 (s, 1H, NH), δ 10.52 (s, 1H, SH).
5-(3-(4-methoxyphenylamino) benzofuran-2-yl)-1, 3, 4-oxadiazole-2-thiol (2h):
Yield: 76%; m.p. 199-200°C. Anal. Calcd. For C17H13N3O3S: C, 60.15; H, 3.80; N, 12.35%. Found: C, 60.17; H, 3.83; N, 12.38%. IR (KBr, cm−1): 3355 (N-H), 1500 (C-N), 1180 (C=S). 1H-NMR (400 MHz, CDCl3, δ 3.9 (s, 3H, ArOCH3), δ 7.3-7.9 (m, 8H, ArH), δ 9.10 (s, 1H, NH), δ 10.50 (s, 1H, SH).
Biological activity
Anti-inflammatory activity
Compounds were screened for anti-inflammatory activity32-34 by carrageenan induced rat paw edema method. Diclofenac sodium was used as standard. Rats were divided into control, standard and different test groups comprising of six animals in each group. They were fasted overnight with free access to water before experiment. In all groups acute inflammation was induced by sub plantar injection of 0.1 ml of freshly prepared 1% suspension of carrageenan in the right hind paw of the rats and paw volume was measured using Plethysmometer at 0 hr and 3 hr after carrageenan injection. Rats of test groups were administered orally with test compounds 100 mg/kg and the standard group with diclofenac 100 mg/kg orally in 2% aqueous acacia one hr before injection of carrageenan. Control group received only vehicle. Mean difference in paw volume was measured and percentage of inhibition of edema was calculated and given in Table.
Table 1: Biological activity data of the compounds 6a-h
|
Sr. No. |
Compounds |
Dose mg/kg |
Mean difference in paw volume+ S.E after 3 hrs(ml) |
Percentage of inhibition |
|
01 |
Control |
– |
0.90 ± 0.03 |
– |
|
02 |
Diclofenac |
100 |
0.14 ± 0.01* |
84 |
|
03 |
2a |
100 |
0.36 ± 0.02* |
60 |
|
04 |
2b |
100 |
0.40 ± 0.02* |
55 |
|
05 |
2c |
100 |
0.32 ± 0.01* |
64 |
|
06 |
2d |
100 |
0.55 ± 0.05* |
39 |
|
07 |
2e |
100 |
0.29 ± 0.02* |
68 |
|
08 |
2f |
100 |
0.34 ± 0.01* |
62 |
|
09 |
2g |
100 |
0.41 ± 0.05* |
54 |
|
10 |
2h |
100 |
0.38 ± 0.02* |
58 |
Values are expressed as mean ± SEM (n=6)
*P < 0.001 when compared to control group
Results and discussion
All the compounds were characterized by physical data, elemental and spectral analysis. The compounds, 3-arylaminobenzofuran-2-carbohydrazides (1a-h) were synthesized from literature procedure.31 These compounds (1a-h), when refluxed with carbon disulphide in presence of potassium hydroxide in ethanol gave the title compounds. IR spectra of (2a-h) exhibited an absorption bands in the region 3360 cm-1 due to N-H, 1500 cm-1 due to C-N and 1180 cm-1 due to C=S. 1H NMR spectra of (2a-h) revealed a signal at δ 7.0-7.9 as a multiplet corresponding to aromatic protons, a deuterium exchangeable broad singlet at δ 9.08 due to NH proton and a singlet at δ 10.50 corresponding to proton of SH group.
All the new compounds (2a-h) have been screened for anti-inflammatory activity and compared with (1a-h) from literature31 and the results are given in Table. The compounds 2a, 2c, 2f and 2e showed good anti-inflammatory activity. The compounds that bear the substituted aniline moiety gave better anti-inflammatory activity compared to unsubstituted aniline. Thus conversion of carboxylic acid functionality to oxadiazole in benzofuran analogs of anthranilic acid gives better anti-inflammatory activity.31
In conclusion, several benzofuran analogs of fenamates were synthesized. These compounds contain benzofuran moiety instead of benzene moiety which is present in fenamates. Further the carboxylic acid moiety was converted to the oxadiazole. The synthesized compounds were tested for anti-inflammatory activity by carrageenan induced rat paw edema method. Some compounds showed very good activity as compared to diclofenac sodium which was used as a standard. From the comparison of (1a-h) and (2a-h) it has been found that conversion of the carboxylic acid moiety to five membered heterocyclic ring increases the anti-inflammatory activity.
Acknowledgement
The author Balasaheb Y. Mane is thankful to BCUD, University of Pune, Pune (MS) India, for providing BCUD Research grant.
References
- Winder C. W., Wax J. and Welford M., J. Pharmacol. Exp. Ther., 148(1965).
- Winder C. W., Wax J., Serrano L., Jones E. M. and Mc Phee M. L., Arthritis. Rhem., 6, 36(1963).
- Winder C. W., Wax J., Scherrer R. A., Jones E. M. and Short F. W., J. Pharmacol. Exp. Ther., 138, 405(1962).
- Preeti R., Srivastava V. K. and Kumar A., Indian J. Chem., 42B, 1729(2003).
- Kaltenbronn J. S., Scherrer R. A., Shork F. W., Jones E. M., Beatty H. R., Saka M. M., Winder C. V., Wax J. and Williamson W. R. N., Arzeim. Forsch. / Drug. Res., 33, 621(1983).
- Perumattam J. J., Chem. Abstr. CA 201802r.
- Sharma S., Srivastava V. K. and Kumar A., Eur. J. Med. Chem., 37, 689(2002).
- Kumar A., Jaju B. P. and Sinha J. N., Indian J. Pharm. Sci., 52(6), 257(1990).
- Abou K. M., Lashine S., Abdulla E. S., Abou T. N. and Amer M. Z., J. Pharm. Sci., 2(1), 140(1993).
- Boschelli D. H., Connor D. T., Bornemeier D. A., Dyer R. D., Kennedy J. A., Kuipers P. J., Okonkwo G. C., Schrier D. J. and Wright C. D., J. Med. Chem., 36, 1802(1993).
- Goel B., Ram T., Tyagi R., Bansal A., Kumar A. and Mukherjee D., Eur. J. Med. Chem., 34(3), 544(1999).
- Sahin G., Palaska E. and Ozlap M., Farmaco., 57(7), 539(2002).
- Dhiman A. M., Wadodkar K. N. and Patil S. D., Indian J. Chem., 40B, 636(2001).
- Hiremath S. P., Sonar V. N., Sekhar K. R. and Purohit M. G., Indian J. Chem., 28B, 626(1989).
- Mashooq A. B., Khan S. A. and Siddiqui N., Indian J. Chem., 14B, 271(2005).
- Priya V. F. and Balakrishna K., Indian J. Chem., 44B, 1456(2005).
- Hansong C., Zhengming Li. and Yufeng, J. Agric. Food. Chem., 48, 5312(2000).
- Xia-Juan Z., Lu-Hua L., Gui-Yu J. Z. and Zi-Xing, J. Agric. Food. Chem., 50, 3757(2002).
- Saad H., Indian J. Chem., 35B, 980(1996).
- Shetgiri N. P. and Nayak B. K., Indian J. Chem., 44B, 1267(2005).
- Ram V. J., Indian J. Chem., 27B, 825(1998).
- Misra U., Hikari A., Saxena A. K., Gurutu S. and Shankae K., Eur. J. Med. Chem., 31, 629(1996).
- Omar F. A., Mahfouz N. M. and Rahman M. A., Eur. J. Med. Chem., 31, 819(1996).
- Mohd A. and Shikha K., Indian J. Heterocyclic Chem., 14, 51(2004).
- Mahadevan K. M., Vaidya V. P. and Vagdevi H. M., Indian J. Chem., 42B, 1931(2003).
- Vagdevi H. M., Latha K. P., Vaidya V. P., Vijaya K. M. L. and Pai K. S. R., Indian J. Pharm Sci., 63(4), 286(2001).
- Kumaraswamy M. N. and Vaidya V. P., Indian J. Heterocyclic Chem., 14, 193(2005).
- Basavaraj P., Vaidya V. P., Mahadevan K. M. and Latha K. P., Indian J. Chem., 44B, 1446(2005).
- Mahajan S. B., Sangapure S. S. and Agasimundin Y. S., Curr. Sci., 45, 722(1976).
- Vaidya, V. P. and Agasimundin, Y. S., Indian J. Chem., 432(1983).
- Mane B. Y., Agasmundin Y. S. and Shivakumar B., Indian J. Chem., 49B, 264(2010).
- Banerjee S., Tapas K. S., Mandal S., Chandra D. P. and Sikdar S., Indian J. Pharmacol., 32, 21(2000).
- Winter C. A., Risely E. A. and Nuss C. W., Proc. Soc. Exp. Med., 111, 544(1962).
- Al-Howiriny T. A., Al-Sohaibani M. O., El-Tahir K. H. and Rafatullah S., J. Natl. Remedies., 3/1, 54(2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.









