New Visible Spectrophotometric Method for the Determination of Cefixime Trihydrate in Pharmaceutical Formulations
S.Imam pasha*, Shravan kumar.A, K. Sravanthi, G. Srinika and V. Nikhila
*Department of Analytical Chemistry, Global College of Pharmacy, Moinabad, R.R.Dist, A.P, (India ).
A simple, sensitive and economical spectrophotometric method was developed for the determination of cefixime trihydrate in pharmaceutical formulations. This method is based on the formation of pink colored chromogen complex by the reaction of drug with ferric chloride and 2, 2 bipyridyl, which absorbs maximally at 520 nm. Beer’s law is obeyed at a concentration range of 1-10 mcg/ml for method. This method has been successfully applied for the assay of the drug in pharmaceutical formulations.
KEYWORDS:cefixime trihydrate; ferric chloride; 2, 2 bipyridyl; Spectrophotometry
Download this article as:| Copy the following to cite this article: Pasha S. I, kumar S, Sravanthi A. K, Srinika G, Nikhila V. New Visible Spectrophotometric Method for the Determination of Cefixime Trihydrate in Pharmaceutical Formulations. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Pasha S. I, kumar S, Sravanthi A. K, Srinika G, Nikhila V. New Visible Spectrophotometric Method for the Determination of Cefixime Trihydrate in Pharmaceutical Formulations. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=24058 |
Introduction
Suprax (cefixime) is a oral suspension, a semi synthetic cephalosporin antibiotic for oral administration.chemically , it is (6R,7R) 7- [2- (2-amino-4-thio-zolyl) glyoxylamido]- 8- oxo-3- vinyl 5-thia-1-azabicyclo[4.2.0] oct-2-ene-2carboxylic acid.cefixime is a broad spectrum antibiotic belongs to third generation cephalosporins. cefixime shows the antibiotic activity by disrupting the synthesis of the peptidoglycan layer of bacterial cell wall. It is effective against both gram positive and gram negative bacteria, so it widely used to treat infections caused by bacteria such as pneumonia, bronchitis, gonorrhea and ear, lung, throat and urinary tract infections.
The drug has been determined by variety of analytical techniques such as high performance liquid chromatography assay with 1,2,4 triazole and mercury chloride[Jun Haginaka and Junko Wakai Analyst, 1985, 110, 1277-1281] , spectroflourimetric study catalyzed by metal ions[P. Gutiérez Navarro, A. El Bekkouri and E. Rodriguez Reinoso Analyst, 1998, 123, 2263-2266], Determination in fermentation media by high-performance liquid chromatography using pre-column derivatisation with 1-hydroxybenzotriazole [Ajit J.Shah, Maxwell W. Adlard and Geoffrey Holt Analyst, 1988, 113, 1197-1200] , Study of spectrophotometric and mercurimetric methods[B. Nowak and H. Wollmann Pharmazie, 1987, 42(12), 862-863], Determination of cefixime in the presence of cloxacillin[A. O. Akanni and J. S. K. Ayim,Department of Pharmaceutical Chemistry, University of Ibadan, Ibadan, Nigeria],Simultaneous spectrophotometric and volumetric determinations[Qureshi SZ, Qayoom T, Helalet MI,Department of Chemistry, Analytical Research Laboratory, Aligarh Muslim University, India.]
The estimation of cefixime was carried out using different methods like spectrophotometric determination of cefixime [J. W. G. Smith, G. E. de Grey and V. J. Patel Analyst, 1967, 92, 247-252], quality control assay [L.A.Okoro E.N.Ejike], Copper(II) complexation with
cefixime[S.V.Lapshin and V.G.Aleksee], determination of spectrophotmetric method with pyrocatechol violet [Amin AS], department of Chemistry, Faculty of Science, Benha University, Benha, Egypt.],Spectrophotometric determination of some cephalosporins with ammonium vanadate.[ Ibrahim el-SA, Beltagy YA, El-Khalek ], Studies on readymix suspension of cefixime trihydrate[Jafar m.*, Aejaz a. Vol 2, Suppl 2, 2010].Different spectrophotmetric have been recommended which include Reaction of hydrochloric acid and potassium iodate followed by na2so4[analytical abstracts 1997], reaction of borate buffer with methanolic chloranil[analytical abstracts 1998], analytical investigation using paramolybdate anion[P.B.Issopoulos,J.Pharm,Biomed,analysis,1998], spectrophotometric method by the reaction of Ce(III) ions complexed with arsenazo III [analytical abstracts 2001],
This paper describes simple and sensitive spectrophotometric method. it includes formation of pink colored complex by the reaction of drug with ferric chloride and 2, 2 bipyridyl, which absorbs maximally at 520 nm.
Materials and Methods
Apparatus
Ultraviolet-Visible-Spectrometer SHIMADZU-1700 with 1 cm matched quartz cells was used for all spectral measurements.
Reagents and standards:
All the chemicals used were of analytical grade.
1). 2, 2 bipyridyl AR grade(2%W/V): 780mg of 2, 2 bipyridyl is dissolved in 100 ml of methanol AR grade.
2). Ferric chloride hexahydrate AR grade(0.2%W/V): 405mg of ferric chloride is dissolved in 100 ml of distilled water.
Procedure
Preparation of standard solution of cefixime trihydrate
Standard stock solution was prepared by dissolving 100mg of cefixime trihydrate in 100 ml of distilled water ,sonicate for 15 min from these aliquots of standard solution taken to prepare 1,2,3,4,5,6,7,8,9,10µg/ml with dilution.
Method :Recommended procedure for the determination of cefixime trihydrate in bulk drug
Aliquots of working sample of drug containing (10-50 mcg/ml ) is transferred into a series of 10ml graduated test tubes. To each test tube 2ml of 2%W/V(0.2560 M) solution of 2, 2 bipyridyl and 0.3 ml of 0.2% (0.012 M) solution of ferric chloride is added. These test tubes along with the blank were heated at a temperature of 700 c for 15 minutes. After heating these test tubes are cooled at room temperature and the volume is made up to 10ml using distilled water.The absorbance of the pink colored chromogen was measured at a maximal wavelength of 520nm against a reagent blank and the concentration was measured using calibration curve.
Procedure For The Assay of Cefixime Trihydrate In Pharmaceutical Formulations
The methods was extended for the determination of cefixime Trihydrate from cefixime formulations. The total contents of 20 cefixime tablets were of powder and the powder equivalent to 10mg was dissolved in 50 ml of dstilled water. and the volume if made to 100ml with distilled water. The above solution was further diluted and analyzed as described, in the above mentioned method for bulk drug. The procedure was repeated three times with cefixime formulations.
Results and Discussion
Iron(Fe) exihibits variable valency and exisists as ferrous (FeII) and ferric (FeIII) salts. Ferrous (Fe II) salts acts as a reductant and involved in complex formation with 2,2 bipyridyl which have a tendency to get oxidized.
Drug when reacted with known amount of iron (FeIII) undergoes oxidation to give reduced form of ferric (FeIII) i.e. ferrous (FeII) ion which has a tendency to give coloured complex with 2,2 bipyridyl.
Reaction Mechanism
The ferrous (FeIII) ion formed by the oxidation of drug undergoes reaction with 3 molecules of 2, 2 bipyridyl to form light pink coloured tris complex
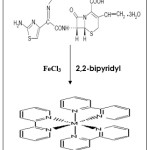 |
Scheme 1 Click here to View scheme |
Conclusion
A simple visible spectrophotometric method for the determination of cefixime trihydrate in pure and its dosage forms was developed. The absorbance of the chromogen was measured at maximum absorbance of 510nm against the corresponding reagent blank. The method is found to be simple, precise, economic, and less time consuming. The method has also been statistically evaluated and the results obtained are accurate, precise and free from the interferences of other additives present in the formulation.
Acknowledgement
Global college of pharmacy, Moinabad,R.Rdist for providing research facilities.
Mr.K.Ramakrishna,Quality control department manager,Endoven pvt limted ,Balanagar,Hyderabad for providing the sample of pure cefixime trihydrate.
References
- Martindale,the complete drug reference,34th edition, royal pharmaceutical society of great Britain, the pharmaceutical press, London,172, (2005).
- Alfred Goodman,Gilman,Joel G.hardman and Limbird,10th edition,Mc graw-hill medical Publishing divison,New delhi,1211,(2001).
- Wilson and Gisvold’s,John H.Block and John
- M.Beale,organic and medicinal pharmaceutical chemistry, Lippincott Williams and wilkins,351-west Camden street,baltimere,331, (2004).
- David A.Williams,Thomas.L.Lemke,Foye’s principles of medicinal chemistry,5th edition, Lippincott Williams and wilkins,351-west Camden street,baltimere,849. (2007).
- Y.R.Sharma, Organic company LTD, New delhi,8, (2002).
- S.M.Khopkar,Basic concepts of analytical chemistry,new age international publishers,New delhi,249. (2008).
- Skoog,west,holler,creuch,estern press pvt.ltd, Banglore,20, (2004).
- Naveen kumar G.Sand Harish K.H,spectrophotometric determination of nebivolo hydrochloride in bulk and in pharmaceutical dosage forms,Indian Drugs.2011,48(4),41-43.

This work is licensed under a Creative Commons Attribution 4.0 International License.









