The One-Pot Synthesis of Pyrano[2,3-d]pyrimidinone Derivatives with 1,4-diazabicyclo[2.2.2]octane in Aqueous Media
Javad Azizian1*, Abolghasem shameli2, Saeed Balalaie3, Mohammad Mehdi Ghanbari4, Shahab Zomorodbakhsh5, Mahdieh Entezari5, Saleh Bagheri2 and Ghasem Fakhrpour2
1Department of Chemistry, Science and Research Branch, Islamic Azad University, P.O. Box 19395-1775, Tehran, Iran.
2Department of Chemistry, Omidyeh Branch, Islamic Azad University, Omidyeh, Iran.
3Peptide Chemistry Research Center, K. N. Toosi University of Technology, P. O. Box 15875-4416, Tehran, Iran.
4Department of Chemistry, Sarvestan Branch, Islamic Azad University, Sarvestan, Iran.
5Department of Chemistry, Mahshahr Branch, Islamic Azad University, Mahshahr, Iran.
1,4-diazabicyclo[2.2.2]octane (DABCO) was used as a catalyst for one-pot, three-component condensation reactions consisting of aromatic aldehydes, malononitrile and thiobarbituric acid in aqueous ethanol at room temperature. This method has the advantages of a simple operation,mild reaction conditions, high yields, by using a less toxic and low cost chemical as a catalyst.
KEYWORDS:thiobarbuturbic acid; malononitrile; DABCO; Aldehyde; Catalyst
Download this article as:| Copy the following to cite this article: Azizian J, shameli A, Balalaie S, Ghanbari M. M, Zomorodbakhsh S, Entezari M, Bagheri S, Fakhrpour G. The One-Pot Synthesis of Pyrano[2,3-d]pyrimidinone Derivatives with 1,4-diazabicyclo[2.2.2]octane in Aqueous Media. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Azizian J, shameli A, Balalaie S, Ghanbari M. M, Zomorodbakhsh S, Entezari M, Bagheri S, Fakhrpour G. The One-Pot Synthesis of Pyrano[2,3-d]pyrimidinone Derivatives with 1,4-diazabicyclo[2.2.2]octane in Aqueous Media. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23888 |
Introduction
Owing to their pharmacological activity, Barbituric (BA) and Thiobarbituric (TBA) acids, as well as their various substituted derivatives, are very important compounds in biological chemistry and medicine. Their biological activity is mainly related to tautomerism and acid-base equilibria and, in turn, to the nature of substituents [1-3]. It is known that barbituric acid itself has no affect on the central nervous system [4], however it is a precursor to medical barbiturates which can be lethal in excessive amounts [5]. Other work has shown that in mice, barbituric acid will cause liver and kidney weight increase [6]. Barbituric acid is also a precursor to derivates that have been shown to have antibacterial activity [7,8] and for tumor inhibitory agents [9]. Therefore, determination of trace amounts of barbituric acid is very important both in studies of biological and industrial processes.
 |
Scheme 1 Click here to View Scheme |
aqueous media. Due to the diverse biological properties of this compound class, there is a widespread interest in their synthesis. Compounds with an uracil moiety antitumor, antibacterial, antihypertensive, vasodilator, bronchiodilator, hepatoprotective, cardiotonic, and antiallergic activities. Some of them exhibit antimalarial, antifungal analgesics, and herbicidal properties [10–17].
Previous methods for the synthesis of 7-amino-6-cyano-5-aryl-5H-pyrano[2,3-d]pyrimidin-(1H, 3H)-2,4-diones have been reported in which a two-component reaction between arylidene malononitrile with barbituric acid occurred under harsh thermal conditions [18]. Also, a microwave-assisted one-pot three-component cyclocondensation of barbituric acids, benzaldehyde derivatives, and alkylnitrile in the absence or presence of triethylamine Diammonium hydrogen phosphate (DAHP) [19-20] has been reported. These methods exhibit some disadvantages such as: harsh conditions, long reaction times, low yields, and effluent pollution.
Experimental
All of the chemical materials used in this work were purchased from Merck and Fluka and used without further purification. Melting points were determined with an Electro thermal 9100 apparatus and were uncorrected. IR spectra were obtained on an ABB FT-IR (FTLA 2000) spectrometer. 1HNMR and 13C NMR spectra were recorded on a Bruker DRX-500 AVANCE at 500 and 125MHz (respectively) using TMS as internal standard and DMSO-d6 as solvent. Mass spectra data were obtained using a GC-MS Hewlett Packard (EI, 20 e V) instrument.
Synthesis 7-Amino-6-cyano-5-(Aryl)4-oxo-2-thioxo-5H-pyrano[2,3-d] pyrimidinone
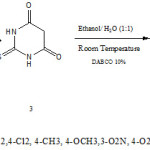
A solution of aromatic aldehyde 1 (1 mmol), malononitrile 2 (1.2 mmol ), barbituric acid 3 (1 mmol), and 1,4-diazabicyclo[2.2.2]octane (DABCO) (10 mol %) in H2O (10 ml) and EtOH (10 ml) was stirred at room temperature for 2 h . After completion of the reaction, the solid product was collected by filtration and purified by washing with aqueous ethanol.
7-Amino-6-cyano-5-(4-bromophenyl)-4-oxo-2-thioxo-5H-pyrano[2,3-d]pyrimidinone (4a): White color powder, m.p. 236◦C (dec.). IR (KBr) (νmax/cm−1): 3370 (NH2), 3189 (NH), 2220 (C≡N), 1684 (C=O), 1567cm−1. 1H-NMR: δ=4.26 (s, 1H, H-5), 7.20 (d, 2H, 3 JHH= 8.2Hz, H-Ar), 7.48 (d, 2H, 3JHH=8.2Hz, H-Ar), 7.86 (brs, 2H, NH2), 12.45 (brs, 1H, NH), 13.66 (brs, 1H, NH) ppm. 13C-NMR: δ=35.28 (CH), 58.48 (C-CN), 82.76 (C), 118.99 (C≡N), 120.00 (C-Br), 129.91 (2 CH), 132.19 (2 CH), 132.74 (C), 143.04 (C-NH2), 157.42 (C=O), 160.34 (C), 173.97(C=S) ppm.
7-amino-6-cyano-5-(3-chlorophenyl)-4-oxo-2-thioxo-5H-pyrano [2,3-d]pyrimidinone (4b): White color powder, m.p. 237–238◦C. IR (KBr) (νmax/cm−1): 3373, 3196 (NH2), 3199, 3061 (NH), 2203 (C≡N), 1683 (C=O), 1569cm−1. 1H-NMR: δ=4.29 (s, 1H,H-5), 7.19 (brs, 2H,NH2), 7.21–7.35 (m, 4 H, H-Ar), 12.45 (brs, 1H, NH), 13.50 (brs, 1H, NH).13C-NMR: δ=35.46 (CH), 57.95 (C-CN), 92.79 (C), 118.96 (C≡N), 126.46 (CH), 127.50 (CH), 128.3 (CH), 130.24 (CH), 132.95 (C-Cl), 146.10 (C), 151.96 (C-NH2),157.57 (C=O), 160.31 (C), 173.98 (C=S) ppm. Mass: (C14H9ClN4O2S) m/z(%)= 334 (7, M2+), 332 (20, M+), 266 (100), 268 (43), 231(56), 221 (64).
7-Amino-6-cyano-5-(2,3-dichloro phenyl)-4-oxo-2-thioxo-5H-pyrano [2,3-d]pyrimidinone (4c): White color powder, m.p. 257–258◦C. IR (KBr) (νmax/cm−1): 3460, 3316 (NH2), 3172,3064 (NH), 2190 (C≡N), 1671 (C=O), 1574cm−1. 1H-NMR:δ=4.85 (s, 1H, H-5), 7.28–7.35 (m, 4H, H-Ar and NH2),7.51 (d, 1H, 3JHH=7.4Hz, H-Ar), 12.46 (brs, 1H, NH),13.70 (brs, 1H, NH) ppm. 13C-NMR: δ=34.03 (CH), 57.45(C-CN), 93.29 (C), 119.35 (C≡N), 129.20 (2 CH), 130.00(CH), 131.26 (C-Cl), 132.59 (C-Cl), 144.00 (C), 153.10 (CNH2),158.57 (C=O), 160.93 (C), 174.87 (C=S) ppm.Mass:(C14H8Cl2N4O2S)m/z(%)=370 (0.1, M4+), 368 (0.4,M2+), 366 (0.7, M+), 265 (100), 267 (41), 222 (18), 206(82), 187(23).
7-amino-6-cyano-5-(3-nitrophenyl)-4-oxo-2-thioxo-5Hpyrano[2,3-d] pyrimidinone (4d): White color powder, m.p. 233.5–234◦C.IR (KBr) (νmax/cm−1): 3420, 3311 , 3197 (NH2), 2201(C≡N), 1687 (C=S), 1574 (C=O) cm−1. 1H-NMR: δ=4.52(s, 1H, H-5), 7.31 (brs, 2H, NH2), 7.60 (t, 1H,3JHH=7.0Hz, H-Ar), 7.76 (d, 1H, 3 JHH=7.0Hz, H-Ar),8.10 (d, 2H, 3JHH=1.2Hz, H-Ar), 12.46 (brs, 1H, NH),13.70 (brs, 1H, NH) ppm.13C-NMR: δ=35.48 (CH), 58.26(C-CN), 93.32 (C), 119.65 (C≡N), 122.92 (CH), 123.19(CH), 130.74 (CH), 135.52 (CH), 146.62 (C), 148.61 (CNO2),152.85 (C-NH2), 158.49 (C=O), 161.14 (C), 174.88(C=S) ppm. Mass: (C14H9N5O4S) m/z(%)=343 (2, M+),277 (100), 260 (23), 230 (17), 179 (49).
7-Amino-6-cyano-5-(4-nitrophenyl)-4-oxo-2-thioxo-5Hpyrano[2,3-d]pyrimidinone (4e):White color powder, m.p. 235–236◦C. IR (KBr) (νmax/cm−1): 3366(NH2), 3190 (NH), 2197(C≡N), 1687 (C=O), 1569cm−1. 1H-NMR: δ=4.46 (s, 1H,H-5), 7.31 (brs, 2H, NH2), 7.55 (d, 2H, 3 JHH=8.3Hz,H-Ar), 8.15 (d, 2H, 3 JHH=8.3Hz, H-Ar), 12.47 (brs, 1H,NH), 13.50 (brs, 1H, NH) ppm. 13C-NMR: δ=35.61 (CH),57.28 (C-CN), 92.43 (C), 118.80 (C≡N), 123.60 (2 CH),129.30 (2 CH), 146.49 (C), 151.16 (C-NO2), 152.09 (CNH2),157.57 (C=O), 160.31 (C), 174.09 (C=S) ppm.
7-Amino-6-cyano-5-(4-trifluoromethyl)-4-oxo-2-thioxo-5H-pyrano[2,3-d]pyrimidinone (4f): Pale yellow powder, m.p. 239–240◦C. IR (KBr) (νmax/cm−1): 3383 (NH2), 3186 (NH), 2198 (C≡N), 1697 (C=O), 1671, 1637cm−1. 1H-NMR: δ=4.35(s, 1H,H-5), 7.26 (brs, 2H,NH2), 7.48 (d, 2H, 3 JHH=8.1Hz, H-Ar), 7.66 (d, 2H, 3 JHH=8.1Hz, H-Ar), 12.46 (brs, 1H,
NH), 13.70 (brs, 1H, NH) ppm. 13C-NMR: δ=36.03 (CH), 58.57 (C-CN), 93.54 (C), 119.69 (C≡N), 126.08 (2 CH), 126.24 (2 CH), 129.34 (C), 130.98 (CF3), 149.02 (C), 152.82 (C-NH2), 158.34 (C=O), 161.09 (C), 174.88 (C=S) ppm. Mass: (C15H9F3N4O2S)m/z(%)=366 (28, M+), 300 (100), 231 (43), 221 (75), 202(57).
7-Amino-6-cyano-5-(2, 4-dichlorophenyl)-4-oxo-2-thioxo-5H-pyrano[2,3-d]pyrimidinone (4g): White color powder, m.p. 238.5–239.5◦C. IR (KBr) (νmax/cm−1): 3388 (NH2), 3306, 3198(NH), 2196 (C≡N), 1687 (C=O), 1647cm−1. 1H-NMR:δ=4.75 (s, 1H, H-5), 7.25 (brs, 2H, NH2), 7.33 (d, 2H, 3 JHH=7.9Hz, H-Ar), 7.38 (d, 1H, 3 JHH=7.9Hz H-Ar),12.54 (brs, 1H, NH), 13.64 (brs, 1H, NH) ppm. 13C-NMR:δ=38.69 (CH), 57.63 (C-CN), 88.01 (C), 119.43 (C≡N),128.47 (CH), 129.60 (CH), 132.81 (CH), 134.05 (C-Cl),140.91 (C-Cl), 150.37 (C-NH2), 153.67 (C=O), 158.75 (C), 163.16 (C=S) ppm.
Results and discussion
Herein we report a simple synthesis of 7-amino-6-cyano-5-aryl-4-oxo-2-thioxo-5H-pyrano[2,3-d]pyrimidinones as a domino Knoevenagel–Michael condensation [21], catalyzed by 10% DAB-CO in aqueous media at room temperature (Scheme 1).Although we have not yet established the mechanism of the one-pot reaction between benzaldehyde derivatives,malononitrile and thiobarbitoric acid in the presence of DABCO, a possible explanation is presented in Scheme 2 We suggest that DABCO is an effective catalyst for the formation. The higher reactivity of the iminium group is utilized to facilitate Knoevenagel condensation between aryl aldehyde 1 and malononitrile 2, which proceeds via intermediate 5 and, after dehydration, olefin 7 is produced. DABCO also catalyzes the generation of a proposed carbonium thiobarbituric acid and this intermediate adds to olefin 6 to generate 4, after proton transfer, tautomerization andnhydrolysis of intermediate 8 (Scheme 2).
The structures of compounds 4(a-g) were deduced from their 1H NMR, 13C NMR and IR spectral data and their molecular weight confirmed bymass spectrometry. 1HNMR and 13C NMR spectroscopy were especially useful to elucidate the structures of products. Thus, all of the products exhibited a singlet peak at about δ =4.22–4.85ppm for H-5 in the 1H NMR spectra, and also a distinctive signal at δ =35–36ppm for C-5 in the 13C NMR spectra. The mass spectra of
The proposed mechanism for the synthesis of 7-Amino-6-cyano-5-(Aryl)4-oxo-2-thioxo-5H-pyrano[2,3-d] pyrimidinone in aqueous media catalyzed by 1,4-diazabicyclo[2.2.2] octane (DABCO).
these compounds detected the expected molecular ion signals. Selected spectroscopic data have been given in general procedure section.
Table 1 shows the results obtained in the reaction of a series of representative aldehydes with malononitrile and thiobarbitoric acid. The effect of substituents on the aromatic ring did not show special effects in terms of yields under these reaction conditions.
Table 1: Synthesis with 10% mol Catalyst DABCO
| Product | Ar | yield |
| 6a | 4-Cl-C6H4 | 89 |
| 6b | 3-Cl-C6H4 | 83 |
| 6c | 2,3-Cl2-C6H3 | 91 |
| 6d | 3-O2N-C6H4 | 96 |
| 6e | 4-O2N-C6H4 | 92 |
| 6f | 4-CF3-C6H4 | 95 |
| 6g | 2,3-Cl2-C6H3 | 85 |
Conclusion
We have developed an easier, practically convenient, novel, ecologically safe method for the synthesis of pyrano[2,3-d]pyrimidinone derivatives using a green chemistry protocol. The use of DABCO as a green catalyst not only gave high yields of products but also provided a procedure that does not use harmful organic solvents
References
- G.G. Hawley, The Condensed Chemical Dictionary, 10th ed, VAN Company, New York, 1981, p. 104.
- R. Acheson, Introduction to Chemistry of Heterocyclic Compounds, Interscience Publishers, New York, 1967, p.339.
- D. Brown, R. Evans, T. Batterham, The Pyrimidines Supplements, Wiley Interscience, New York, 1970, p. 199.
- W. Wesson, D. Smith (Eds.), Barbiturates: Their Use, Misuse, and Abuse, Science Press, New York, 1977, p. 18.
- H. Matther (Ed.), Acute Barbiturate Poisoning, Excerpta Medica, Amsterdam, 1971, p. 14.
- A. Reid, M.J. Turnbull, Arch. Int. Pharmacodyn. Ther. 1974, 211, 49.
- P. Prisyazhnik, G. Palii, Y. Volydnskii, A. Lopushanskii, E. Opanasenko, Khim. Farm. Zh. 1976, 10, 46.
- M. Omar, Egypt J. Pharm. Sci. 1998, 38, 281.
- A. Kreutzberger, Arzneim.-Forsch. 1978, 28, 1684.
- D. Fenn (ed) (1994) The pyrimidines. Wiley, New York; D. Heber, C. Heers, U. Ravens (1993) Positive inotropic activity of 5-amino-6-cyano-1,3-dimethyl-1,2,3,4-tetrahydropyrido[2,3-d]pyrim idine-2,4-dione in cardiac muscle from guinea-pig and man. Part 6: compounds with positive inotropic activity. Pharmazie 48:537–541
- a)E.M. Griva, S. Lee, C.W. Siyal, D.S. Duch, C.A. Nichol, J. Med. Chem. 1980, 23:327–329; b) G.L. Anderson, J.L. Shim, A.D. Broom. J. Org. Chem, 1976, 41:1095–1099
- M.M. Ghorab, A.Y. Hassan, Phosphorus Sulfur Silicon Relat Elem, 1998, 141:251–261
- S. Furuya, T. Ohtaki Pyrido[2, 3-d]pyrimidines and their use as endothelin antagonists. Eur Patent (1994) 608565
- W.J. Coates, Preparation of pyrimidopyrimidine derivatives useful as bronchodilators, vasodilators, antiallergic. Eur Patant (1990) 351058
- N. Kitamura, A. Onishi, Pyrimidopyrimidinedione derivatives and their use antiallergic agent. Eur Patant (1984) 163599
- J. Davoll, J. Clarke, E.F. Elslager, J Med Chem. 1972, 15:837–839
- G. Levitt, Herbicidal sulfonamides. US Patant (1982) 4339267
- Y. Gao, S.J. Tu, T. Li, X. Zhang, S. Zhu, F. Fang, D. Shi, Synth Commun, 2004, 34:1295–1299
- I. Devi, B.S.D. Kumar, P.J. Bhuyan, Tetrahedron Lett, 2003, 44:8307–8310
- R.J. Lewis, Hawley’s condensed chemical dictionary 13th edn. Von Nostrand Reinhold, New York (1997)
- a) S. Abdolmohammadi, S. Balalaie, Tetrahedron Lett, 2007, 48 ,3299–3303 b) S. Balalaie, S.Abdolmohammadi, B. Soleimanifard, Helvetica Chimica Acta, 2009, 92(5), 932–936 c) S. Abdolmohammadi, S. Balalaie, ChemInform, 2007, 38 (33) d) S. Balalaie, S. Abdolmoh-ammadi, H. R. Bijanzadeh, A. M. Amani, Molecular Diversity ,2008, 12, 2, 85-91.
- L.T. Tietze, G .Brasche, K.M. Gericke Domino reactions in organic synthesis. Wiley-VCH, Weinheim (2006) 542–565

This work is licensed under a Creative Commons Attribution 4.0 International License.









