Removal of Toxic Azo Dyes from Wastewater Using Bottom Ash - Equilibrium Isothermal Modeling
Arti Malviya¹ and Dipika Kaur²
¹Department of Engineering Chemistry, Lakshmi Narain College of Technology, Bhopal (India). ²Department of Engineering Chemistry, Symbiosis Institute of Technology, Pune (India).
This article describes the use of a power plant waste-Bottom Ash, as effective adsorbent for the removal of toxic Azo dyes Carmoisine and Chrysoidine Y from their aqueous solution. The paper incorporates comparative study and discussion of the adsorption characteristics of these dyes on the economically effective adsorbent with special emphasis on the effects of time and temperature. The characterizations of the adsorbent have been carried out by standard analytical techniques. The adsorption behavior of the dyes has been studied using some well-known Isotherms like Freundlich, Langmuir, Tempkin and Dubinin-Radushkevich adsorption isotherm models. The experimental results confirmed that both the dyes were potentially adsorbed by the power plant waste.
KEYWORDS:Bottom Ash; Langmuir Isotherm; Dubinin-Radushkevich; Freundlich; Tempkin; monolayer adsorption
Download this article as:| Copy the following to cite this article: Malviya A, Kaur D. Removal of Toxic Azo Dyes from Wastewater Using Bottom Ash - Equilibrium Isothermal Modeling. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Malviya A, Kaur D. Removal of Toxic Azo Dyes from Wastewater Using Bottom Ash - Equilibrium Isothermal Modeling. Available from: http://www.orientjchem.org/?p=23500 |
Introduction
Increase in the industrialization has increased the pollution of water bodies. Azo dyes are the class of dye characterized by the presence of highly stable -N=N- group and are one of the widely used dye in various sectors like textile, leather, rubber, plastic, food industries etc. The colored wastewater not only damages the aesthetic nature of water and but is also capable of potentially destroying the ecological balance of the water system.1 This is attributed to the fact that the water-soluble azo dyes are highly resistant towards their biodegradation due to complex structures and high thermal and photo stability. Thus the release of azo dyes into the environment is of concern due to toxic, mutagenic and carcinogenic characteristics2 of these dyes and their biotransformation products.3 Azo dyes are detected of causing chromosomal damage4 and also reductive cleavage of azo groups forms carcinogenic aromatic amine.5 Removal of colored dyes from wastewaters is thus a major environmental issue.
Amongst the various techniques adapted for the removal of dyes from wastewater, adsorption6,7 has been found to be an effective method due to its efficiency, simplicity of design, and applicability. Various researchers have studied the adsorption of dyes on various types of materials. A number of low-cost adsorbents have been examined for the treatment of wastewaters, such as saw dust,8 castor seed shell9 coconut waste,10 kaolinite11 etc.
In the present investigations Bottom Ash is utilized as an adsorbent for removal of hazardous coloring agent, Carmoisine and Chrysoidine Y from wastewaters (Table 1). Being stable the effluent containing Carmoisine and Chrysoidine is difficult to treat in environment system. It has been found that the oral administration of dye in animals results in liver-cell adenomas, carcinomas and leukemia.12 Both the dyes may also undergo reduction followed by chain of reactions leading to formation of toxic components. The present paper focuses on the comparative isothermal characteristics of the concerned dyes.
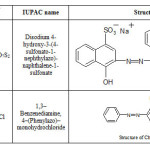 |
Table 1: Characteristics of the Dyes Click here to View table |
Materials and Methods
Both the dyes were obtained from M/s Merck and the stock solutions were prepared in doubly distilled water. The greyish black granular and porous Bottom Ash was obtained from Bharat Heavy Electrical Limited, Bhopal. The absorbance measurements were carried out on UV/Vis Spectrophotometer model no. 117 (M/s Systronics, Ahmedabad, India) over the wavelength range 400 – 600 nm. Scanning Electron Microscopy was carried out using Philips SEM 501 Electron Microscope and Philips X-ray Diffractophotometer was employed for X-ray measurements. Quantasorb Model QS-7 surface area analyzer, mercury porosimeter and specific gravity bottles determined physical characteristics of the adsorbents.
Material Development
The adsorbent under study that is Bottom Ash was first washed and dried material was then treated with Hydrogen peroxide for about one day. Chemically treated adsorbent was then washed thoroughly and dried in an oven for optimum time to remove moisture and was further activated in a furnace at 500oC and then sieved.
Adsorption Studies
In this experiment, a series of 100mL volumetric flasks containing 25mL of adsorbate solutions at different concentrations were employed at predetermined pH. An appropriate amount of adsorbents of a particular particle size was added into each flask separately. The solution was then filtered and the filtrate was analyzed for the final concentration of the dye spectrophotometrically by measuring absorbance at respective lmax of the dyes under study.
Results and Discussion
Characterization of Adsorbents
Standard chemical as well as analytical techniques were employed to ascertain the properties of adsorbents. Some important physical properties of Bottom Ash like; density, porosity, and surface area, were also determined as per standard procedures (Table 2 and Table 3).
Table 2: Chemical Constituents of Bottom Ash
|
Bottom Ash |
|
|
Constituents |
Percentage by weight |
|
SiO2 |
45.4 |
|
CaO |
15.3 |
|
Moisture |
15.0 |
|
Al2O3 |
10.3 |
|
Fe2O3 |
9.7 |
|
MgO |
3.1 |
|
Na2O |
1.0 |
Table 3: Physical Characteristics of Bottom Ash
|
Parameters |
Bottom Ash |
|
Surface Area (cm2.g–1) |
870.5 |
|
Density (g.mL–1) |
0.6301 |
|
Porosity (%) |
46 |
|
Loss on Ignition (%) |
1.14 |
Effect of Particle Size
The adsorption capacity of Bottom Ash was investigated for their wide range of particle sizes viz. 0.425 mm to 0.08 mm. With increase in the mesh size adsorption capacity was found to increase which led to the conclusion that with increase in mesh size that is increase in the surface area of the adsorbent and thus adsorption increases.
Effect of Contact Time
Contact time study was carried out in order to determine the equilibrium time for maximum uptake of the dye. Experiments were carried out for different contact time with a fixed adsorbent dose of 0.05g and at different temperatures (30, 40 and 50oC). Within one hour of contact, greater than 50 % of the dyes get adsorbed over Bottom Ash at 30oC. The plot of time against initial concentration (Figure 1) reflects rapid adsorption of dyes by the adsorbents and thereafter, the adsorption rate decreases gradually and the adsorption reaches to optimal removal within 4 to 5 hours for both the dyes over Bottom Ash. After reaching the saturation value a continuous and smooth curve is obtained. The rate of removal of dye decreases with time may be due to aggregation of dye molecules around the adsorbent particles.
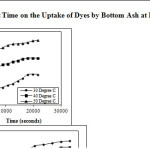 |
Figure 1: Effect of Contact Time on the Uptake of Dyes by Bottom Ash at Different Temperatures Click here to View figure |
 |
Table 1: Click here to View table |
Adsorption Isotherms
In the present study, the comparative adsorption of Carmoisine and Chrysoidine Y onto the Bottom Ash was studied at different temperatures (30, 40 and 50oC) and the applicability of the above mentioned isotherms were examined.
Freundlich Isotherm
The Freundlich Isotherm is a model that considers heterogeneous adsorption energies on the adsorbent surface.13,14
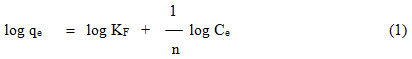
Where, qe is the amount adsorbed (molg–1), Ce is the equilibrium concentrationof the adsorbate (molL–1). KF and n, the Freundlich constants are related to adsorption capacity and adsorption intensity, respectively. The linear plots of log Ce versus log qe (Figure 2 clearly reveals that adsorption of dye on Bottom Ash Freundlich model. The values of regression coefficients suggest better fit of Freundlich isotherm for the Carmoisine –Bottom Ash system. The values of KF and n were derived from the intercepts and slope, for both the adsorption system are listed in Table 4.
Table 4:Constant parameters calculated for various adsorption models at different temperatures for Carmoisine and Chrysoidine Y
|
Freundlich Constants |
Carmoisine |
Chrysoidine Y |
||||
|
30 oC |
40 oC |
50 oC |
30 oC |
40 oC |
50 oC |
|
|
n |
0.783 |
0.735 |
0.768 |
1.074 |
1.104 |
1.207 |
|
KF |
2.931 |
16.780 |
18.724 |
3.41 |
2.27 |
2.61 |
|
Langmuir Constants |
|
|||||
|
Qo × 10–5 (mol/g) |
1.210 |
1.600 |
1.780 |
7.27 |
5.85 |
10.6 |
|
b× 104 (L/mol) |
0.911 |
1.382 |
2.312 |
1.613 |
1.000 |
0.249 |
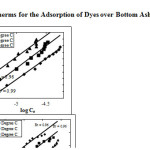 |
Figure 2: Freundlich Isotherms for the Adsorption of Dyes over Bottom Ash at Different Temperatures |
Langmuir Isotherm
Langmuir sorption model assumes that uptake of adsorbate occurs on a homogenous surface by monolayer adsorption without any interaction between the adsorbed ions. Also, all the binding sites on the surface are having uniform energies of adsorption [15, 16]. The mathematical form of Langmuir equation can be expressed as,
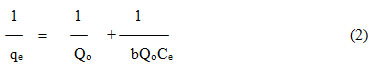
Where, qe is the amount of Chrysoidine Y adsorbed (molg–1), Ce is the equilibrium molar concentration of the dye (molL–1), Qo is the maximum adsorption capacity (molg–1) and b is the energy of adsorption (Lmol–1). The slope and intercept obtained from the plot of 1/qe against 1/Ce gives the Langmuir constants at each temperature. The straight line obtained further confirms that the Langmuir Isotherm is followed in the adsorption process (Figure 3). The monolayer adsorption capacity, at 30oC was found to be 1.20 × 10–5 molg–1 and 7.27 ×10–5 molg–1 for Carmoisine- Bottom ash and Chrysoidine-Bottom Ash, respectively. The values of calculated parameters for Langmuir are presented in Table 4. The dimensionless adsorption intensity is calculated using the following relation,
 |
Table 1: Click here to View table |

Where, b denotes the Langmuir constant and Co the initial concentration (Weber and Chakrabarti, 1974).17
Tempkin Model and Dubinin-Radushkevich (D-R) Isotherm Model
The Tempkin isotherm assumes that the heat of adsorption of all the molecules in the layer decreases linearly with coverage due to adsorbent–adsorbate interactions [18].
Both the dyes adsorption curve suggests the poor fit of the isotherm to the adsorption process. The D-R model is based on the heterogenous characteristics of the adsorbents [19] and is expressed as follows:
![]()
Where, Cads is the amount of dye adsorbed per unit weight of adsorbent (molg–1), Xm is the maximum adsorption capacity (molg–1), b is the activity coefficient (mol2 J–2) related to mean adsorption energy and Î is the Polanyi potential. The slope of straight-line graph of lnCads against Î 2 (Figure 4) gives activity coefficient and intercept yields adsorption capacity. The good fit of the Dubinin–Radushkevich (D–R) isotherm (all R2 values >0.92) suggest that this isotherms are more appropriate for the adsorption process for both the adsorption systems and greatest at 30oC. The calculation of the mean sorption energy helps in distinguishing between chemical and physical adsorption. For E<8 kJmol−1, physisorption dominates the sorption mechanism. If E is between 8 and 16 kJ mol−1, chemisorption is the governing factor. The values of E was found to be 7.91 kJ mol–1 and 8.27 kJ mol−1 for Carmoisine – Bottom Ash and Chrysoidine- Bottom Ash respectively, suggesting that the adsorption process was dominated by chemisorptions.
The present investigation highlights the adsorptive characteristics of toxic azo dyes onto an effective adsorbent Bottom Ash. In this study the maximum sorption of both the dyes was reported in the particle size range of 0.15 – 0.08 mm (100 BSS Mesh size). Results of sorption energy obtained from D-R isotherm shows the chemisorptions nature of the sorption process as the value of the sorption energy falls between 8 and 16 kJ mol−1. The study leads to the conclusion that both the dyes can be potentially removed from waste water using Bottom Ash.
References
- Muthukumar, M., Karuppiah, M. T. and Raju, G. B., Electrochemical removal of CI Acid orange 10 from aqueous solutions, Separation and Purification Technology, 55, 198–205, 2007.
- Aboel-Zahab, H., El-khyat, Z., Sidhom, G., Awadallah, R., Abdel-al, W., and Mahdy, K., Physiological effects of some synthetic food colouring additives on rats, Bollettino Chimico Farmaceutico, 136, 615–627, 1997.
- Lin, Y., Leu, J., Kinetics of reactive azo-dye decolorization by Pseudomonas luteola in a biological activated carbon process, Biochemical Engineering Journal, 39, 457–467, 2008.
- Vajnhandl, S., Le Marechal, A. M., Case study of the sonochemical decolouration of textile azo dye Reactive Black 5, Journal of Hazardous Materials, 141, 329–335, 2007.
- Biswas, M.M., Taylor, K.E., Bewtra, J.K., and Biswas, N., Enzymatic treatment of sulphonated aromatic amines generated from reductive degradation of reactive azo dyes, Water Environment Research, 79, 351–356, 2007.
- Mohan, S. V., Ramanaiah, S.V., and Sarma, P.N., Biosorption of direct azo dye from aqueous phase onto Spirogyra sp. I02: Evaluation of kinetics and mechanistic aspects, Biochemical Engineering Journal, 38, 61–69, 2008.
- Chatterjee, S. Chatterjee, S., Chatterjee, B. P, and Guha, A.K., Adsorptive removal of congo red, a carcinogenic textile dye by chitosan hydrobeads: Binding mechanism, equilibrium and kinetics, Colloids and Surfaces A: Physicochem. Eng. Aspects, 299, 146–152, 2007.
- Memon, S.Q., Memon, N., Solangi, A.R., Memon, and J., Sawdust: A green and economical sorbent for thallium removal, Chemical Engineering Journal, 140, 235 – 240, 2008.
- Oladoja, N.A., Aboluwoye, C.O., Oladimeji, Y.B., Ashogbon, A.O., and Otemuyiwa, I.O., Studies on castor seed shell as a sorbent in basic dye contaminated wastewater remediation, Desalination, 227, 190–203, 2008.
- Hameed, B.H., Mahmoud, D.K, and Ahmad, A.L., Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (Cocos nucifera) bunch waste, Journal of Hazardous Materials, 158, 65–72, 2008.
- Unuabonah, E. I., Adebowale, K. O., and Dawodu, F. A ., Equilibrium, kinetic and sorber design studies on the adsorption of Aniline blue dye by sodium tetraborate-modified Kaolinite clay adsorbent, Journal of Hazardous Materials, 157, 397–409, 2008.
- Cartwright, R.A., Robinson, M.R.G. ,. Glashan, R.W, Gray, B.K., Hamilton-Stewart, P. , Cartwright, S.C., and Barnham-Hall, D., Does the use of stained maggots present a risk of bladder cancer to coarse fishermen? Carcinogenesis, 4, 111—113, 1983.
- Faria, P.C.C., Orfao, J.J.M., Figueiredo, J.L., and Pereira, M.F.R., Adsorption of aromatic compounds from the biodegradation of azo dyes on activated carbon, Applied Surface Science, 254, 3497–3503, 2008.
- Ofomaja, A. E., Ho, Y., Equilibrium sorption of anionic dye from aqueous solution by palm kernel fibre as sorbent, Dyes and Pigments, 74, 60–66, 2007.
- Huang, Y., Hsueh, C., Huang, C., Su, L., and Chen, C., Adsorption thermodynamic and kinetic studies of Pb(II) removal from water onto a versatile Al2O3-supported iron oxide, Separation and Purification Technology, 55, 23–29, 2007.
- Weber, and Chakrabarti, Pore and solid diffusion models for fixed bed adsorbers, Journal of American Institute of Chemical Engineering, 20, 228–238, 1974.
- Srivastava, V.C., Mall, I. D., and Mishra, I. M., Adsorption thermodynamics and isosteric heat of adsorption of toxic metal ions onto bagasse fly ash (BFA) and rice husk ash (RHA), Chemical Engineering Journal, 132, 267–278, 2007.
- Sarı, A., and Tuzen, M., Biosorption of cadmium (II) from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies, Journal of Hazardous Materials, 157, 448–454, 2008.
- Gunay A., Arslankya E., and Tosun I., Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption Equilibrium Kinetics, Journal of Hazardous Materials, 146, 362—371, 2007.

This work is licensed under a Creative Commons Attribution 4.0 International License.









