Amino Acid Composion and Sugar Analysis of Mcj Lectin
Ajay Pratap Singh* and K. D. Sexena
Department of Chemistry Bareilly College, Bareilly - 243 001 (India)
Momardica charantia L.var. Jhalri (MCJ) is the member of lectins from the family Cucurbitaceae. For further characterization MCJ lectin was analyzed for amino acid identification and sugar analysis by paper chromatography in the present paper. The technique reported the presence of total 18 amino acids with large content of Serine and Glutamic acid. The same technique revealed the presence of glucose, mannose and hexosamine. The total hexose sugar by Orcinol- sulphuric acid method and total hexosamine by Elson and Moragn method was estimated to be 6.81mg and 5.037mg respectively per 100mg of MCJ lectin. The good content of carbohydrate in MCJ lectin being glycoprotein in nature was considered as a major and crucial factor for apparent high mol. Wt. of MCJ lectin.
KEYWORDS:Lectin; Paper chromatography; Sugar; Glycoprotein
Download this article as:| Copy the following to cite this article: Singh A. P, Sexena K. D. Amino Acid Composion and Sugar Analysis of Mcj Lectin. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Singh A. P, Sexena K. D. Amino Acid Composion and Sugar Analysis of Mcj Lectin. Available from: http://www.orientjchem.org/?p=23485 |
Introduction
Lectins are proteins which have specific binding affinity for carbohydrate moiety and can thus interact with saccharides.1 These proteins can agglutinate cells and combine with glycocomponents of the cell surface causing a number of biological properties.2Lectins are a very widespread group of plant proteins because sources are almost endless. Plant lectins have been isolated from all parts of plant but largely from seeds. Besides plants they have been found in bacteria, fungi, snails and even mammals. Most plant lectins contain covalently bound carbohydrate moieties such as mannose, galactose, N-actylglucosamine, L-arabinose.3-6 These proteins are glycoprotein lectins. In each carbohydrate unit of a glycoprotein lectin a single sugar residue is linked glycosidically to the side chain of an amino acid. The linkage is either N-glycosidic via the amide nitrogen of an asparagine residue or O-glycosidic via the hydroxyl of serine, threonine, hydroxylysine, or hydroxyproline.3-7 In the previous paper8 MCJ lectin was isolated, purified and detected for biological activity. In the present study identification amino acids and sugar analysis of MCJ lectin has been performed using paper chromatography9
Material and Methods
Identification of amino acids:
For this purpose first Purified MCJ lectin was hydrolyzed with 6N Hydrochloric acid at 110°C for 12hrs. The hydrolyzates were concentrated under vaccum over sodium hydroxide pallets. The residue was dissolved in a small amount of triple distilled water. The hydrolyzates along with standards were applied to the Whatman No3 chromatographic paper 36X56 cm with the help of fine capillaries. After air drying, Chromatographic paper was saturated with butanol vapours and was carefully hanged in cuvettes to which solvent prepared by mixing Butanol (AR, Qualigen Fine chem. Pvt. Ltd), acetic acid (AR, s.d. fine chem. Pvt. Ltd) and water in ratio 4:1:15(v/v/v) was added carefully and allowed to flow uniformly. The chromatograms so obtained were dried, sprayed with ninhydrin solution and heated at 50°C in an oven for 10 min when coloured spots were developed and were compared with standards. MCJ lectin was subjected to alkaline hydrolysis with 5N NaOH at 100°C in the same way as in acid hydrolysis to detect Tryptophan in it.
Sugar analysis of MCJ lectin
The procedure used was similar as used in identification of amino acids. For getting individual sugars, first hydrolysis was carried out with 4M hydrochloric acid for 4hrs at 100°C [10]. After single dimension chromatographic run of hydrolyzates, it was sprayed with aniline phthalate. Spots of brown colour were developed indicating the presence of sugars.
Estimation of total hexose sugar in MCJ lectin. It was carried out by Orcinol- Sulphuric method11 using glucose (AR, s.d. fine chem. Pvt. Ltd) as standard .The absorbance readings were taken at 540nm by “Systronic Spectrophotometer169” against water blank.
Estimation of total hexosamine in MCJ lectin: Total hexosamine was estimated by method of Elson Morgan and Rondle12-14 using glucosamine hydrochloride as standard.
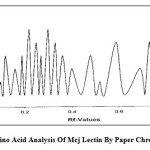 |
Figure 1: Amino Acid Analysis Of Mcj Lectin By Paper Chromatography |
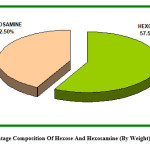 |
Figure 2: Percentage Composition Of Hexose And Hexosamine (By Weight) In Total Sugar
|
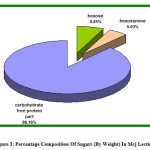 |
Figure 3: Percentage Composition Of Sugars (By Weight) In Mcj Lectin |
Result and Discussion
Identification of amino acids: Acid hydrolysis of MCJ lectin led to the formation of brown colouration of the hydrolyzates. This was probably due to the formation of humin by the side reactions between amino acids and sugars.15 The study of amino acids by paper chromatography revealed the presence of following 18 amino acids: –
Lysine, Histidine, Arginine, Asparatic acid, Serine, Glycine, Threonine, Glutamic acid, Alanine, Proline, Tryosine, Methionine, Valine, Cysteine, Isoleucine, Phenyl alanine, Leucine and Tryptophan.
Tryptophan was detected separately by alkaline hydrolysis because it is destroyed in acidic-hydrolysis with 6NHCl. The colour intensities of the spots on the paper chromatogram were evaluated by scanning in densitometer at 509 nm (fig.a) and indicated that the present protein was rich with Serine and Glutamic acid (maximum intensity) while poor with Tryptophan and Cysteine (minimum intensitiy) amino acids. MCJ lectin was found to contain Methionine which generally remains absent in plant lectins.
Sugar analysis of MCJ lectin: The technique used showed the presence of Glucose and Mannose as hexoses and glucosamine as mere sugar among hexosamines in MCJ lectin. In Orcinol-Sulphuric acid method reaction used for the quantitative estimation was based on heating of glycoprotein (MCJ lectin) with strong acid to form furfuraldehyde. The reaction of this degradation product with orcinol forms a coloured complex, having the characteristic absorption at 420 nm and 540 nm.16 The optical density at 540 nm in this case was preferred because at this wavelength the other sugars present did not interfere the hexose determination. The experiment showed that the total hexose present was 8.096 mg per 100 g seeds of Momardica charantia L. var. Jhalri. The total hexose percentage in protein isolated was 6.81% i.e. 6.81 mg hexose per 100 mg of MCJ lectin isolated.
The quantitative estimation of hexosamine by Elson and Morgan method concluded that there was 5.984 mg glucosamine per 100 g of MCJ seeds. The total Glucosamine (Hexosamine) percentage in protein isolated was 5.037% i.e. 5.037 mg Glucosamine per 100 mg of MCJ lectin isolated. MCJ lectin being a glycoprotein in nature consists of carbohydrate moieties as prosthetic group. It is evident that there is a good content of carbohydrate moieties (11.84%w/w) in MCJ lectin isolated (Fig. b& c). Due to presence of carbohydrate moieties, SDS (Sodium Dodecyl Sulphate) finds less space on MCJ lectin compared with polypeptides of equal mass [17] to bind with it. This causes slow migration of MCJ lectin on SDS-Gel electrophoresis. This explains apparent high molecular weight of 128kDa of MCJ lectin as determined by SDS-PAGE in the previous paper. The work is further progressive for structural elucidation, detailed biological activity and metal ion detection in MCJ lectin.
Acknowlegment
The authors thank principal, Bareilly College Bareilly for providing research facilities, Dr Praveen Singh, Senior scientist Biophysics Section and Late Dr. N. Ahmad, Biochemistry Division, IVRI Bareilly for availing chemical and study material, and Prof. Y. D. Sharma Head Biotech, AIIMS Delhi for their valuable assistance and suggestions.
References
- Grubhoffer, L., Hypsa., V., Parasite 4: 203-216(1997).
- Sharon, N., Lis, H., in: Lectin, Chapman & Hall, New Yark, 2-65 (1989).
- Dorland, L., Van Halbeek, H., Vliegenthart, J. F. G., Lis, H., Sharon, N., J. Biol. Chem., 256:7708-7711(1981).
- Lis, H. and Sharon, N., In Biology of Carbohydrates, ed. V. Ginsburg, P. W. Robbins., New York. Wiley, 2: 1-85 (1984).
- Sharon, N., ‘Complex Carbohydrate. Their Chemistry, Biosynthesis and Functions’, Addition- Wesley, ReadingMA, 1-32 and 178-180 (1975).
- Brewer, M and Scott, T., ‘Concise Encyclopedia of Biochemistry’, Walter de Gruyter, Berlin, 189-190 (1983).
- Clair R Cole and Christopher A Smith, Biochemical Education, 17(4)):179-189 (1989).
- Singh., A. P., Sexsena, K. D., Bioscience Biotechnology Research Asia, 8(2):841-844 (2011)
- Leisegang, R. E., J. Analyt. Chem., 126:172 (1943).
- Chandransekaran, E. V., Bemiller, J. N., Methods Carohydr. Chem., 8: 89-96 (1980).
- Winzler, R. J., Methods Biochem. Anal., 2: 279-311 (1955).
- Elson, L. A., Morgan, W. T. J., Biochem. J., 27: 1824 (1933).
- Morgan, W. T. J., Elson, L. A., Biochem. J., 28:288 (1934).
- Rondle, C. M. J., Morgan, W. T. J., Biochem. J., 61: 586-589 (1955).
- Narasimhareddy, S. B., Swamy, P. M., “Science Spectrum.” 13 (6): 360-361(1976).
- Dische, Z. J., Biol. Chem., 181:379(1949).
- Leach, B. S., Collawn, J. F. and Fish, W. W., Biochemistry, 19: 5734-5741(1980).

This work is licensed under a Creative Commons Attribution 4.0 International License.









