Nmr Studies of Bh3-Attaching in the Zigzag and Armchair Bn Nanotubes: A Dft Study
Reza Soleymani 1*, Maryam Karimi-Cheshmeh Ali2 and Nahid Niakan3
1Young Researchers Club, Shahre-rey Branch, Islamic Azad University, Tehran (Iran). 2Department of Chemistry, Mahshahr Branch, Islamic Azad University, Mahshahr (Iran). 3Department of Chemistry, Shahre-rey Branch, Islamic Azad University, Tehran (Iran).
This article investigates the structure of armchair (3, 3) and zigzag (6, 0) boron nitrogen nanotubes (BNNTs) affected by addition of a Borane group (BH3) by performing density functional theory (DFT) calculation at B3LYP levels of theory and 6-31G(d) basis set. The changes of the nuclear magnetic resonance (NMR) parameter of these structures were calculated by GIAO method implemented in the Gaussian 09W program of package. The results indicated that the addition of BH3 to nanotubes affected the values of isotropic chemical shift (CSI) and anisotropic chemical shift (CSA) parameters. The addition of BH3 also affected the values of bond length and bond angle. The results revealed that the attachment of BH3 group to the surface of nanotubes had the potential to increase the chemical shift of the nuclei directly linked to Borane molecules.
KEYWORDS:BNNTs; CSA; CSI; DFT; NMR; BN Nanotubes
Download this article as:| Copy the following to cite this article: Soleymani R, Ali M. K. C , Niakan N. Nmr Studies of Bh3-Attaching in the Zigzag and Armchair Bn Nanotubes: A Dft Study. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Soleymani R, Ali M. K. C , Niakan N. Nmr Studies of Bh3-Attaching in the Zigzag and Armchair Bn Nanotubes: A Dft Study. Available from: http://www.orientjchem.org/?p=23431 |
Introduction
Boron nitride compound, with chemical formula BN, is made up of boron and nitrogen atoms 1, 2. This compound is not found in nature and can only be constructed from boric acid or boron trioxide in laboratory. It has hexagonal or cubic structure. The hexagonal form (h-BN) is among the most stable and softest BN polymorphs and is used as a lubricant or an additive to cosmetic products 3-7. BNNTs are very similar to carbon nanotubes (CNTs) and, therefore, can form graphite like hexagonal layers. BN is chemically inerter than carbon especially in high temperatures. However, the electronic properties of BNNTs are less adjustable than that of CNTs. BNNTs are also better field emitters than CNTs. BNNTs like nanotubes produced from tungsten and sulfur or tungsten and selenium has less flexibility and elasticity in comparison with CNTs. However, BN nano-mesh has a two dimensional structure which is not only stable to decomposition under vacuum, some gases and some liquids but also resistant to high temperatures of about 900 °C 7-10. Limitations in production and application of CNTs have triggered a deluge of interest in BNNTs. This material has a layered structure so that each boron atom is bonded to a nitrogen atom. This material has specific properties such as having superior mechanical properties (young’s modulus 1.18 TPa) and high heat resistance and remaining semi-conductive. This material is employed in production of fiber. BNNTs fibers are utilized in production of commercial textile, military uniforms, and solar cells 10-16. Many theoretical and experimental researches were conducted to study the synthesis of BNNTs. This material was theoretically predicted in 1994 but its synthesis was experimentally realized in 1995 in laboratory with arc-charging method using BN electrode. Later, other methods such as arc-melting, high temperature chemical reaction, carbon nanotube templates, and laser ablating were reported for synthesis of this material 16-22. Recently, Zeng et al. has investigated the effect of NH3 on these nanotubes 23. Zahedi et al. has also conducted theoretical researches on (10, 0) nanotubes investigating the effect of NH3 on NMR parameters of these structures 24. Here, regarding the wide application and great importance of this material, armchair and zigzag BNNTs were studied to investigate how their properties would be affected by addition of a BH3 group. For this reason, NMR dependant parameters such as CSI and CSA were calculated. Finally, some of their structural parameters were evaluated.
Computational Details
All the computations were carried out by performing DFT calculation at B3LYP and 6-31G(d) levels using the Gaussian 09W program of package 25. This was performed on a personal computer (Pentium® 4 CPU 1.70 GHz and 4.00 GB of RAM) with Microsoft Windows 7 system. All the calculations were carried out in gas phase at atmospheric pressure and a temperature of 298 K. NMR parameters were calculated by GIAO method 26, 27. The armchair (3, 3) and zigzag (6, 0) models of BNNTs were designed using Gauss View and optimized through semi-empirical method Austin Model 1 (AM1) 28. Each nanotube had a length of 10 Å. The values of CSI and CSA parameters were determined through relations 1 and 2 29-31. CS tensors in the principal axes system (PAS) (σ33> σ22> σ11)
CSI (ppm) = (σ11 + σ22 + σ33)/3 (1)
CSA (ppm) = σ33 − (σ11 + σ22)/2 (2)
Result And Discussion
After the final optimization of the structure of nanotubes the values of chemical shifts and structural parameters were evaluated. In order to do so, the structural parameters of armchair (3, 3) and zigzag (6, 0) models of BNNTs were investigated under perfect and BH3 attached states.
Geometrical parameter
Bond length and angle are considered as the main structural parameters. In this article, bond lengths, expressed in angstrom unit, and bond and dihedral angles, specified in degrees, were determined and the effect of adding a BH3 group to the surface of BNNTs was investigated (Fig 1-5).
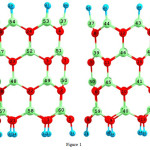 |
Figure 1: 2D views of the perfect in (6, 0) zigzag model of BNNTs. Click here to View table |
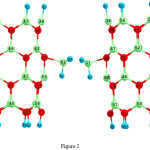 |
Figure 2: 2D views of the BH3-attach in (6, 0) zigzag model of BNNTs. Click here to View figure |
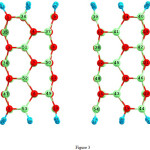 |
Figure 3: 2D views of the perfect in (3, 3) armchair model of BNNTs. Click here to View figure |
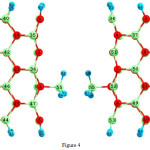 |
Figure 4: 2D views of the BH3-attach in (3, 3) armchair model of BNNTs. Click here to View figure |
 |
Figure 5: Behind 2D views of the perfect in (3, 3) armchair and (6, 0) zigzag models of BNNTs. Click here to View figure |
Bond length in (3, 3) armchair BNNTs
The results of armchair (3, 3) BNNTs indicated that addition of BH3 group induced changes in the bond length in proportion with the position which BH3 took on the ring. Accordingly, all bond lengths would change by addition of BH3 groups and the changes would be bigger in bonds which were closer to the BH3 bonding area. For example, N2-B55 direct bond induced the attachment of a BH3 group to the surface of the nanotube. The biggest changes in bond length were observed in N2-B36 bond (1.458 Å to 1.496 Å), N2-B47 bond (1.465 Å to 1.504 Å), and N2B52 (1.453 Å to 1.509 Å). Other bond lengths were also subjected to similar changes but their changes were smaller (Table 1).
Table 1: Bond length (Angstrom) of perfect and BH3-attach in (3, 3) armchair model of BNNTs.
|
Bond length |
Armchair Perfect |
Armchair BH3 attach |
Bond length |
Armchair Perfect |
Armchair BH3 attach |
|
N1-H23 |
1.011 |
1.011 |
N13-B39 |
1.471 |
1.472 |
|
N1-B34 |
1.420 |
1.418 |
N13-B41 |
1.448 |
1.447 |
|
N1-B35 |
1.461 |
1.460 |
N14-H24 |
1.011 |
1.011 |
|
N2-B36 |
1.458 |
1.496 |
N14-B40 |
1.420 |
1.419 |
|
N2-B47 |
1.465 |
1.504 |
N14-B41 |
1.461 |
1.460 |
|
N2-B52 |
1.453 |
1.509 |
N15-B43 |
1.448 |
1.446 |
|
N3-B35 |
1.465 |
1.463 |
N15-B48 |
1.466 |
1.466 |
|
N3-B36 |
1.458 |
1.451 |
N15-B54 |
1.471 |
1.473 |
|
N3-B51 |
1.453 |
1.448 |
N16-B43 |
1.465 |
1.466 |
|
N4-B34 |
1.471 |
1.474 |
N16-B45 |
1.458 |
1.459 |
|
N4-B37 |
1.448 |
1.448 |
N16-B46 |
1.453 |
1.451 |
|
N4-B51 |
1.466 |
1.462 |
N17-B41 |
1.465 |
1.467 |
|
N5-H30 |
1.011 |
1.010 |
N17-B42 |
1.453 |
1.450 |
|
N5-B47 |
1.461 |
1.458 |
N17-B45 |
1.458 |
1.457 |
|
N5-B53 |
1.420 |
1.410 |
N18-B35 |
1.448 |
1.449 |
|
N6-B49 |
1.448 |
1.448 |
N18-B40 |
1.471 |
1.472 |
|
N6-B52 |
1.466 |
1.452 |
N18-B42 |
1.466 |
1.462 |
|
N6-B53 |
1.471 |
1.480 |
N19-H32 |
1.011 |
1.011 |
|
N7-B50 |
1.452 |
1.455 |
N19-B43 |
1.461 |
1.462 |
|
N7-B51 |
1.462 |
1.469 |
N19-B44 |
1.420 |
1.416 |
|
N7-B52 |
1.462 |
1.448 |
N20-B44 |
1.471 |
1.477 |
|
N8-B37 |
1.465 |
1.465 |
N20-B46 |
1.466 |
1.463 |
|
N8-B38 |
1.453 |
1.453 |
N20-B47 |
1.448 |
1.434 |
|
N8-B50 |
1.458 |
1.455 |
N21-B36 |
1.452 |
1.439 |
|
N9-H26 |
1.011 |
1.011 |
N21-B42 |
1.462 |
1.469 |
|
N9-B37 |
1.461 |
1.460 |
N21-B46 |
1.462 |
1.459 |
|
N9-B39 |
1.420 |
1.420 |
H22-B40 |
1.195 |
1.195 |
|
N10-H29 |
1.011 |
1.011 |
H25-B39 |
1.195 |
1.195 |
|
N10-B49 |
1.461 |
1.459 |
H27-B34 |
1.195 |
1.195 |
|
N10-B54 |
1.420 |
1.419 |
H28-B53 |
1.195 |
1.194 |
|
N11-B48 |
1.453 |
1.453 |
H31-B44 |
1.195 |
1.193 |
|
N11-B49 |
1.465 |
1.462 |
H33-B54 |
1.195 |
1.195 |
|
N11-B50 |
1.458 |
1.455 |
B55-H56 |
– |
1.206 |
|
N12-B38 |
1.462 |
1.463 |
B55-H57 |
– |
1.208 |
|
N12-B45 |
1.452 |
1.450 |
B55-H58 |
– |
1.199 |
|
N12-B48 |
1.462 |
1.462 |
N2-B55 |
– |
1.803 |
|
N13-B38 |
1.466 |
1.465 |
– |
– |
– |
Bond length in (6, 0) zigzag BNNTs
The results of zigzag (6, 0) BNNTs were completely similar to the results of armchair (3, 3) BNNTs. Similarly, the biggest changes were observed in bonds which were closer to the BH3 group. In this form, N19-B61 direct bond induced the attachment of a BH3 group to the surface of the nanotube. This factor also resulted in bigger changes in the bonds which contained N19 atom. For example, the biggest changes in bond length were observed in N19-B42 bond (1.458 Å to 1.506 Å), N19-B47 bond (1.457 Å to 1.507 Å), and N19B55 bond (1.450 Å to 1.543 Å). No significant change was observed in other bond lengths (Table 2).
Table 2: Bond length (Angstrom) of perfect and BH3-attach in (6, 0) zigzag model of BNNTs.
|
Bond length |
Zigzag Perfect |
Zigzag BH3 attach |
Bond length |
Zigzag Perfect |
Zigzag BH3 attach |
|
N1-B33 |
1.015 |
1.015 |
N14-B56 |
1.457 |
1.450 |
|
N1-B58 |
1.449 |
1.450 |
N15-B41 |
1.444 |
1.459 |
|
N1-B60 |
1.433 |
1.431 |
N15-B42 |
1.461 |
1.434 |
|
N2-B49 |
1.461 |
1.460 |
N15-B46 |
1.459 |
1.459 |
|
N2-B50 |
1.469 |
1.470 |
N16-B38 |
1.457 |
1.461 |
|
N2-B60 |
1.457 |
1.460 |
N16-B42 |
1.451 |
1.443 |
|
N3-B49 |
1.450 |
1.453 |
N16-B43 |
1.456 |
1.461 |
|
N3-B51 |
1.458 |
1.457 |
N17-H36 |
1.015 |
1.015 |
|
N3-B52 |
1.457 |
1.452 |
N17-B56 |
1.432 |
1.439 |
|
N4-B37 |
1.456 |
1.457 |
N17-B57 |
1.449 |
1.454 |
|
N4-B51 |
1.451 |
1.452 |
N18-B48 |
1.462 |
1.437 |
|
N4-B53 |
1.457 |
1.456 |
N18-B55 |
1.458 |
1.506 |
|
N5-H31 |
1.024 |
1.024 |
N18-B57 |
1.454 |
1.447 |
|
N5-B59 |
1.487 |
1.486 |
N19-B42 |
1.458 |
1.506 |
|
N5-B60 |
1.488 |
1.488 |
N19-B47 |
1.457 |
1.507 |
|
N6-B45 |
1.460 |
1.458 |
N19-B55 |
1.450 |
1.543 |
|
N6-B50 |
1.471 |
1.472 |
N20-B38 |
1.456 |
1.459 |
|
N6-B59 |
1.461 |
1.463 |
N20-B47 |
1.453 |
1.445 |
|
N7-B39 |
1.459 |
1.459 |
N20-B54 |
1.456 |
1.460 |
|
N7-B50 |
1.444 |
1.447 |
N21-H34 |
1.015 |
1.015 |
|
N7-B51 |
1.461 |
1.462 |
N21-B57 |
1.451 |
1.454 |
|
N8-B37 |
1.457 |
1.457 |
N21-B58 |
1.451 |
1.444 |
|
N8-B39 |
1.453 |
1.454 |
N22-B48 |
1.461 |
1.460 |
|
N8-B44 |
1.456 |
1.454 |
N22-B49 |
1.458 |
1.453 |
|
N9-H32 |
1.014 |
1.014 |
N22-B58 |
1.454 |
1.457 |
|
N9-B40 |
1.433 |
1.433 |
N23-B47 |
1.459 |
1.431 |
|
N9-B59 |
1.433 |
1.433 |
N23-B48 |
1.450 |
1.466 |
|
N10-B40 |
1.461 |
1.463 |
N23-B52 |
1.459 |
1.459 |
|
N10-B41 |
1.471 |
1.468 |
N24-B52 |
1.453 |
1.454 |
|
N10-B45 |
1.460 |
1.455 |
N24-B53 |
1.456 |
1.458 |
|
N11-B39 |
1.458 |
1.456 |
N24-B54 |
1.456 |
1.451 |
|
N11-B45 |
1.455 |
1.457 |
H25-B44 |
1.192 |
1.191 |
|
N11-B46 |
1.458 |
1.452 |
H26-B37 |
1.192 |
1.192 |
|
N12-B43 |
1.457 |
1.452 |
H27-B53 |
1.192 |
1.192 |
|
N12-B44 |
1.456 |
1.458 |
H28-B43 |
1.192 |
1.191 |
|
N12-B46 |
1.453 |
1.453 |
H29-B54 |
1.192 |
1.191 |
|
N13-H35 |
1.024 |
1.024 |
H30-B38 |
1.192 |
1.190 |
|
N13-B40 |
1.487 |
1.484 |
B61-N19 |
– |
1.610 |
|
N13-B56 |
1.488 |
1.492 |
B61-H62 |
– |
|
|
N14-B41 |
1.469 |
1.446 |
B61-H63 |
– |
1.197 |
|
N14-B55 |
1.460 |
1.507 |
B61-H64 |
– |
1.196 |
Bond angle and dihedral angle in (6, 0) zigzag and (3, 3) armchair BNNTs
Investigation of bond and dihedral angles in armchair (3, 3) and zigzag (6, 0) BNNTs indicated that addition of BH3 group to the surface of BNNTs did not significantly affect the values of bond and dihedral angles. Specific bonds, which were more affected by the added BH3 group, were studied. The results were of subtle differences. The biggest changes were found among the studied angles in the zigzag form of which B42-N19-B55-N14 dihedral angle recorded the most significant change (166.4 °C to -6.9 °C). The results are reported in Tables 3 and 4.
Table 3: Bond Angle (Degree) and dihedral angle (Degree) of perfect and BH3-attach in (3, 3) armchair model of BNNTs.
|
Bond Angle |
Armchair Perfect |
Armchair BH3 attach |
|
B52-N2-B55 |
– |
105.4 |
|
B36-N2-B55 |
– |
110.7 |
|
B47-N2-B55 |
– |
106.1 |
|
B52-N2-N36 |
112.4 |
108.2 |
|
B36-N2-B47 |
119.2 |
117.2 |
|
B47-N2-B52 |
112.6 |
117.2 |
|
|
|
|
|
Dihedral Angle |
|
|
|
N7-B52-N2-B36 |
12.2 |
17.5 |
|
N7-B52-N2-B47 |
150.4 |
145.6 |
|
N6-B52-N2-B36 |
-149.9 |
-144.9 |
|
N6-B52-N2-B47 |
-11.6 |
-16.8 |
|
B55-N2-B52-N6 |
– |
96.5 |
|
B55-N2-B52-N7 |
– |
31.2 |
|
B55-N2-B36-N3 |
– |
62.9 |
|
B55-N2-B36-N21 |
– |
-135.3 |
|
B55-N2-B47-N20 |
– |
137.9 |
|
B55-N2-B47-N5 |
– |
59.9 |
Table 4: Bond Angle (Degree) and dihedral angle (Degree) of perfect and BH3-attach in (6, 0) zigzag model of BNNTs.
NMR properties
|
Bond Angle |
Zigzag Perfect |
Zigzag BH3 attach |
|
B61-N19-B55 |
– |
76.6 |
|
B61-N19-B47 |
– |
118.6 |
|
B61-N19-B42 |
– |
121.0 |
|
B42-N19-B55 |
117.5 |
117.2 |
|
B47-N19-B55 |
118.3 |
117.9 |
|
B42-N19-B47 |
110.0 |
104.3 |
|
|
|
|
|
Dihedral Angle |
|
|
|
B61-N19-B47-N23 |
– |
108.3 |
|
B61-N19-B47-N20 |
– |
-86.1 |
|
B61-N19-B42-N16 |
– |
85.1 |
|
B61-N19-B42-N15 |
– |
-110.2 |
|
B61-N19-B55-N18 |
– |
-112.7 |
|
B61-N19-B55-N14 |
– |
111.3 |
|
B42-N19-B55-N14 |
166.4 |
-6.9 |
|
B47-N19-B55-N18 |
11.7 |
2.8 |
|
B42-N19-B55-N18 |
147.9 |
128.9 |
|
B47-N19-B55-N14 |
-151.8 |
-133.0 |
The changes of the NMR parameter of these structures were calculated by GIAO method. The structures were optimized and NMR dependent parameters such as CSI and CSA were calculated. The results indicated that addition of BH3 to the structure of BNNTs induced changes in the values of CSI and CSA parameters which affected the chemical properties of the nanotubes.
CSI parameter
3.2.1.1. CSI in armchair (3, 3) BNNTs in perfect and BH3-attach
CSI values indicated that addition of BH3 group induced changes in CSI parameter. The changes were in proportion with the position of BH3 group on the surface of nanotube. Chemical shift of the nucleus of B, N, and H atoms were completely different. The results are reported in Table 5. In perfect state, the chemical shift of N nuclei had a 125 ppm to 144 ppm range and the chemical shift of B nuclei had a 78 ppm to 82 ppm range. The shift ranges were slightly affected by addition of a BH3 group. After addition of a BH3 group, the chemical shift of N nuclei had a 122 ppm to 181 ppm range and the chemical shift of B nuclei had a 78 ppm to 121 ppm range. Comparison of different nuclei showed that addition of a BH3 group would increase the chemical shift range. Nuclei which were adjacent to the external BH3 group showed the highest chemical shift. N2 and B47 had the highest chemical shift (Fig 6).
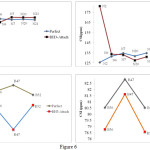 |
Figure 6: Show compared results of various nucleus in perfect and BH3-attach in (3, 3) armchair model of BNNTs Click here to View figure |
Table 5: Isotropic Chemical Shift (ppm) and Anisotropic Chemical Shift (ppm) values for various nucleus of perfect and BH3-attach in (3, 3) armchair model of BNNTs.
|
Perfect |
BH3-Attach |
|||
|
CSI |
CSA |
CSI |
CSA |
|
|
N1 |
143.80 |
115.08 |
143.86 |
115.76 |
|
N2 |
125.72 |
161.67 |
181.18 |
91.04 |
|
N3 |
125.72 |
161.67 |
122.11 |
162.08 |
|
N4 |
131.56 |
160.37 |
132.14 |
158.77 |
|
N5 |
143.80 |
115.08 |
143.63 |
117.96 |
|
N6 |
131.56 |
160.37 |
133.77 |
155.10 |
|
N7 |
134.80 |
159.00 |
133.12 |
162.80 |
|
N8 |
125.37 |
161.50 |
126.34 |
161.62 |
|
N9 |
144.01 |
114.96 |
143.61 |
116.39 |
|
N10 |
144.01 |
114.96 |
144.63 |
114.67 |
|
N11 |
125.37 |
161.50 |
126.02 |
162.21 |
|
N12 |
135.09 |
159.07 |
134.19 |
158.72 |
|
N13 |
131.75 |
160.46 |
131.10 |
160.42 |
|
N14 |
143.87 |
115.05 |
143.88 |
115.53 |
|
N15 |
131.75 |
160.46 |
131.00 |
159.39 |
|
N16 |
125.36 |
161.60 |
126.50 |
161.37 |
|
N17 |
125.35 |
161.60 |
125.42 |
162.09 |
|
N18 |
131.88 |
159.74 |
131.98 |
162.01 |
|
N19 |
143.87 |
115.05 |
143.57 |
116.09 |
|
N20 |
131.88 |
159.74 |
127.69 |
162.66 |
|
N21 |
134.84 |
158.73 |
131.09 |
162.45 |
|
B34 |
78.97 |
55.57 |
79.04 |
55.89 |
|
B35 |
82.83 |
38.10 |
83.13 |
38.57 |
|
B36 |
80.54 |
37.71 |
78.78 |
36.92 |
|
B37 |
82.73 |
38.11 |
83.00 |
37.92 |
|
B38 |
80.46 |
37.65 |
80.45 |
37.95 |
|
B39 |
79.02 |
55.58 |
78.97 |
55.70 |
|
B40 |
78.95 |
55.62 |
78.91 |
55.44 |
|
B41 |
82.82 |
37.96 |
82.70 |
38.21 |
|
B42 |
80.46 |
37.86 |
80.93 |
37.13 |
|
B43 |
82.82 |
37.96 |
82.69 |
38.62 |
|
B44 |
78.96 |
55.62 |
78.95 |
55.75 |
|
B45 |
80.65 |
37.65 |
80.60 |
38.09 |
|
B46 |
80.46 |
37.86 |
81.87 |
36.23 |
|
B47 |
82.83 |
38.11 |
81.62 |
35.55 |
|
B48 |
80.46 |
37.65 |
80.51 |
38.01 |
|
B49 |
82.73 |
38.11 |
82.56 |
38.62 |
|
B50 |
80.56 |
37.89 |
80.74 |
37.92 |
|
B51 |
80.53 |
37.55 |
81.33 |
35.79 |
|
B52 |
80.53 |
37.55 |
78.58 |
36.96 |
|
B53 |
78.97 |
55.57 |
79.68 |
53.24 |
|
B54 |
79.02 |
55.58 |
78.92 |
56.08 |
|
B55 |
– |
– |
121.12 |
44.26 |
CSI in zigzag (6,0) BNNTs in perfect and BH3-attach
Trends in chemical shift of some nuclei are compared in Table 6. Chemical shift of different nuclei in perfect state showed a 94 ppm to 100 ppm range in N nuclei and a 75 ppm to 81 ppm range in B nuclei (Table 6). However, by addition of a BH3 group, the chemical shift of N nuclei changed to a 98 ppm to 219 ppm range and the chemical shift of B nuclei changed to a 73 ppm to 129 ppm range. Nuclei which were more affected by the BH3 group were subjected to further investigation (Fig 7). CSI values for the nuclei of different atoms showed that N19 and B55 recorded the highest shift range.
 |
Figure 7: Show compared results of various nucleus in perfect and BH3-attach in (6, 0) zigzag model of BNNTs. |
Table 6: Isotropic Chemical Shift (ppm) and Anisotropic Chemical Shift (ppm) values for various nucleus perfect and BH3-attach in (6, 0) zigzag model of BNNTs.
|
Perfect |
BH3-Attach |
|||
|
CSI |
CSA |
CSI |
CSA |
|
|
N1 |
147.00 |
103.18 |
148.18 |
102.78 |
|
N2 |
126.37 |
169.36 |
125.43 |
167.57 |
|
N3 |
130.46 |
184.32 |
131.02 |
184.34 |
|
N4 |
100.53 |
225.51 |
99.70 |
224.81 |
|
N5 |
194.29 |
74.91 |
190.49 |
76.38 |
|
N6 |
123.55 |
176.22 |
122.62 |
174.97 |
|
N7 |
129.64 |
182.56 |
128.78 |
181.79 |
|
N8 |
100.31 |
225.60 |
99.31 |
225.18 |
|
N9 |
137.17 |
112.50 |
136.94 |
113.01 |
|
N10 |
123.58 |
176.15 |
126.62 |
178.57 |
|
N11 |
130.21 |
185.10 |
131.31 |
185.22 |
|
N12 |
100.32 |
225.62 |
100.44 |
229.25 |
|
N13 |
194.14 |
75.05 |
198.66 |
74.33 |
|
N14 |
126.36 |
169.32 |
146.16 |
134.95 |
|
N15 |
129.63 |
182.56 |
126.32 |
187.56 |
|
N16 |
100.54 |
225.52 |
99.20 |
221.67 |
|
N17 |
147.06 |
103.17 |
147.03 |
96.18 |
|
N18 |
134.06 |
180.11 |
156.75 |
138.39 |
|
N19 |
130.47 |
184.33 |
219.80 |
32.52 |
|
N20 |
100.24 |
225.85 |
98.76 |
221.35 |
|
162.39 |
94.47 |
163.51 |
92.75 |
|
|
N22 |
134.09 |
180.10 |
137.22 |
179.94 |
|
N23 |
131.38 |
184.48 |
128.61 |
190.18 |
|
N24 |
100.24 |
225.85 |
100.24 |
229.43 |
|
B37 |
75.88 |
52.88 |
75.72 |
53.26 |
|
B38 |
75.96 |
52.83 |
73.81 |
53.13 |
|
B39 |
79.41 |
38.69 |
79.12 |
38.90 |
|
B40 |
75.49 |
43.88 |
75.29 |
42.95 |
|
B41 |
76.72 |
36.62 |
79.43 |
32.83 |
|
B42 |
79.06 |
38.70 |
77.06 |
39.23 |
|
B43 |
75.88 |
52.88 |
76.45 |
53.50 |
|
B44 |
76.05 |
52.89 |
75.98 |
52.42 |
|
B45 |
79.74 |
37.83 |
80.27 |
37.30 |
|
B46 |
79.41 |
38.69 |
80.49 |
38.59 |
|
B47 |
79.35 |
38.53 |
77.52 |
38.88 |
|
B48 |
78.56 |
35.99 |
81.49 |
36.40 |
|
B49 |
78.96 |
36.98 |
79.43 |
36.40 |
|
B50 |
76.71 |
36.63 |
75.95 |
37.20 |
|
B51 |
79.06 |
38.70 |
78.54 |
38.73 |
|
B52 |
79.35 |
38.53 |
80.43 |
38.39 |
|
B53 |
75.96 |
52.84 |
75.93 |
52.38 |
|
B54 |
76.01 |
52.78 |
76.59 |
53.31 |
|
B55 |
78.97 |
37.00 |
113.84 |
24.90 |
|
B56 |
75.71 |
43.16 |
75.21 |
42.91 |
|
B57 |
81.57 |
39.26 |
82.20 |
38.78 |
|
B58 |
81.57 |
39.25 |
82.14 |
38.40 |
|
B59 |
75.48 |
43.90 |
75.43 |
44.43 |
|
B60 |
75.70 |
43.17 |
75.56 |
43.78 |
|
B61 |
– |
– |
129.47 |
14.53 |
CSA parameter
CSA in armchair (3, 3) BNNTs in perfect and BH3-attach
The result of CSA values were the reverse of the results of CSI values. In other words, as CSI values increased CSA values decreased. In perfect state, the chemical shift of N nuclei had a 114 ppm to 161 ppm range and the chemical shift of B nuclei at different parts of the nanotube had a 37 ppm to 55 ppm range. After addition of a BH3 group, the chemical shift of N nuclei had a 91 ppm to 162 ppm range and the chemical shift of B nuclei had a 35 ppm to 56 ppm range. According to Figure 6 the nucleus of N2 and B47 atoms recorded the highest shift range after the addition of a BH3 group.
CSA in zigzag (6, 0) BNNTs in perfect and BH3-attach
In perfect state, the chemical shift of N nuclei had a 74 ppm to 255 ppm range and the chemical shift of B nuclei had a 35 ppm to 52 ppm range. After addition of a BH3 group, the chemical shift of N nuclei changed to a 32 ppm to 229 ppm range and the chemical shift of B nuclei changed to a 14 ppm to 53 ppm range. The nucleus of N19 and B55 atoms had the highest chemical shift range (Fig 7). It was evident that the addition of a BH3 group to the surface of nanotube affected all CSA values. The highest shifts were recorded in the nucleus of atoms directly affected by the BH3 group.
Conclusion
From the investigation of armchair (3, 3) and zigzag (6, 0) BNNTs under perfect and BH3 attached states, it can be concluded that:
The values of CSI and CSA parameters have a regular trend in Boron nitride nanotubes under perfect state. Addition of a BH3 group to the surface of nanotube affects this trend so that each parameter finds a specific value proportionate to the location of Boron, Hydrogen and Nitrogen nuclei.
The values of CSI and CSA parameters record their largest shift in the nucleus of the atoms directly linked to BH3 groups.
Addition of a BH3 group resulted in small changes in the values of bond length, bond angle, and dihedral angle. The changes were more significant in the area directly affected by BH3.
Chemical shift of the nucleus of the BH3 group, attached to the surface of the nanotube, shows different behavior in comparison with the chemical shift of the nucleus of B atom within the nanotube structure. The values of CSI and CSA parameters are usually higher in the nucleus of N atom in comparison with B atoms.
Acknowledgements
This work was supported by Islamic Azad University Shahre-rey branch and Islamic Azad University Mahshahr branch.
References
- M. Kawaguchi et al. Journal of Physics and Chemistry of Solids, 69: 1171(2008).
- M.S. Silberberg, Chemistry: The Molecular Nature of Matter and Change (5th ed.), New York: McGraw-Hill, (2009).
- T.P. Crane, B.P. Cowan, Physical Review B, 62: 11359 (2000).
- R. Zedlitz, Journal of Non-Crystalline Solids, 403: 198 (1996).
- C.H. Henager, Applied Optics, 32: 91 (1993).
- S. Weissmantel, Diamond and Related Materials, 8 : 377 (1999).
- G. Leichtfried et al. “13.5 Properties of diamond and cubic boron nitride”. In P. Beiss et al. Landolt-Börnstein-Group VIII Advanced Materials and Technologies: Powder Metallurgy Data. Refractory, Hard and Intermetallic Materials. 2A2. Berlin: Springer. pp. 118 (2002).
- J.H. Lan et al, Physical Review B, 79: 115401(2009).
- J. Hu, X. Ruan, Y.P. Chen, Nano Letters, 9: 2730 (2009).
- T. Ouyang, Y. Chen, Y. Xie, K. Yang, Z. Bao, J. Zhong. Nanotechnology, 21: 245701(2010).
- R. Haubner, M. Wilhelm, R. Weissenbacher, B. Lux, High Performance Non-Oxide Ceramics II, Series Structure and Bonding, vol. 102, Springer/Heidelberg, Berlin/New York, (2002).
- P.B. Mirkarimi, K.F. McCarty, D.L. Medlin, Mater. Sci. Eng. R, 21: 47 (1997).
- X. Blase, A. Rubio, S.G. Louie, M.L. Cohen, Europhys. Lett, 28: 335 (1994).
- A. Loiseau, F. Willaime, N. Demoncy, G. Hug, H. Pascard, Phys. Rev. Lett, 76: 4737 (1996).
- E. Bengu, L.D. Marks, Phys. Rev. Lett, 86: 2385 (2001).
- V. Nirmala, P. Kolandaivel, J. Mol. Struct. (THEOCHEM), 817: 137 (2007).
- A. Rubio, J.L. Corkill, L. Cohen, Phys. Rev. B, 49: 5081 (1994).
- N.G. Chopra, R.J. Luyken, K. Cherrey, H.C. Respi, M.L. Cohen, G. Louie, Science, 269: 966 (1995).
- T. Hirano, T. Oku, K. Sugannuma, Diamond Relat. Mater, 9: 625 (2000).
- R. Ma, Y. Bando, T. Sato, Chem. Phys. Lett, 337: 61 (2001).
- W. Han, Y. Bando, K. Kurashima, T. Sato, Appl. Phys. Lett, 73: 3085 (1998).
- A. Rubio, Y. Miyamoto, X. Blasé, M.L. Cohen, S.G. Louie, Phys. Rev. B, 53: 4023 (1996).
- Z. Zhou, J. Zhao, Z. Chen, P.R. Schleyer, J. Phys. Chem. B, 110: 25678 (2006).
- E. Zahedi, A. Bodaghi, A. Seif, A, Boshra, Superlattices and Microstructures, 49: 169 (2011).
- M.J. Frisch et al., GAUSSIAN 09. Gaussian, Inc., Pittsburgh, PA, (2010).
- R.S. Drago, Physical Methods for Chemists, second ed, Saunders College Publishing, Florida, (1992).
- U. Haeberlen, in: J.S. Waugh (Ed.), Advances in Magnetic Resonance, Academic Press, New York, (1976).
- R. Dennington, T. Keith, J. Millam, K. Eppinnett, W.L. Hovell, R. Gilliland, GaussView, Version 3.07, Semichem, Inc., Shawnee Mission, KS, (2003).
- K. Wolinski, J.F. Hinton, P. Pulay, J. Am. Chem. Soc, 112: 8251 (1990).
- F.J. London, Phys. Radium, 8: 397 (1937).
- R. Ditchfield, Mol. Phys, 27: 789 (1974).

This work is licensed under a Creative Commons Attribution 4.0 International License.









