Template Synthesis of Macrocyclic Complexes of Bivalent CoII, NiII, PdII,ZnII and CdII Ions with Cyclic Glyoxal Carbohydrazone (1,2,4,5,8,9,11,12-octaazacyclotetradeca-5,7,12,14-tetraene-3,10-dione)
L.K. Mishra1, Madhubala1, R.R. Jha2, Preethyalex2 and Rakeshroshan2*
1Department of chemistry, NIT, Patna, India. 2Department of chemistry, Ranchi University, Ranchi, India.
Article Received on :
Article Accepted on :
Article Published : 31 Dec 2012
Cyclic glyoxalcarbohydrazone[(1,2,4,5,8,9,11,12-octaazacyclotetradeca-5,7,12,14-tetraene-3,10-dione) = (glyozalcarbzH)2] complexes of NiII, PdII, CoII, ZnII and CdII of composition M (glyoxalcarbzH)2X2nH2O (M = NiII, PdII, CoII,ZnII or CdII and X = Cl> , 1/2 SO4-2and n = 0,1 or 4) have been isolated by refluxing appropriate metal salts with glyoxal and carbohydrazide in requisite proportion for 8 to 10hours in aqueous ethanol on steam bath. The refluxate on concentration and cooling in ice gave crystalline precipitate of appropriate complexes. The complex salts are soluble in hot aqueous ethanol and conduct electricity showing 1:2 ionic natures. Except ZnII, CdII and PdII the complexes of CoII and NiII are paramagnetic and their magnetic moment value suggested octahedral geometry. The UVand IR spectral results supported octahedral structure for CoII and NiII while planer for PdII complexes.
KEYWORDS:Cyclic glyoxalcarbohydrazone; bivalent metal complexes
Download this article as:| Copy the following to cite this article: Mishra L. K, Madhubala, Jha R. R, Preethyalex, Rakeshroshan. Template Synthesis of Macrocyclic Complexes of Bivalent CoII, NiII, PdII,ZnII and CdII Ions with Cyclic Glyoxal Carbohydrazone (1,2,4,5,8,9,11,12-octaazacyclotetradeca-5,7,12,14-tetraene-3,10-dione). Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Mishra L. K, Madhubala, Jha R. R, Preethyalex, Rakeshroshan. Template Synthesis of Macrocyclic Complexes of Bivalent CoII, NiII, PdII,ZnII and CdII Ions with Cyclic Glyoxal Carbohydrazone (1,2,4,5,8,9,11,12-octaazacyclotetradeca-5,7,12,14-tetraene-3,10-dione). Available from: http://www.orientjchem.org/?p=22869 |
Introduction
The concept of macrocyclic compound and their metal complex grew rapidly after synthesis of crown ether by Pederson in 19661. The growing application of macrocyclic complexes in transport of alkali metal nutrient to plant kingdom enhanced the interest of Chemist and Biologist to synthesize and characterize the metal complexes with macrocycliccrown ether2-5. In present paper we describe the
preparation and characterization of complexes ofmacrocyclicligand bis(glyoxalcarbohydrazone) with bivalent metal ions.
Experiment
The metal salts, glyoxaldihydrate, hydrazine hydrate, diethylcarbonate and organic solvents were extra pure reagent of E.Merck and AnalR grade chemicals of BDH. Palladium (II) Chloride was obtained from JohnsonMetthey London.Carbohydrazide was prepared by refluxing diethylcarbonate {O=C(OC2H5)} with 98% hydrazine hydrate on steam bath as reported in literature6. The magnetic susceptibility of complexes were determined by Gouy method at room temperature using [Hg{Co(NCS)4}]as standered(χg= 16.24×10-6 CGS.unit at 20oC). The metal content, halogens and sulphate were determined by standard process8. The results of C,H and N were obtained from BIT Mesra. Ranchi.The IR spectra were recorded as KBr disc in the range 450–4000cm-1 onShemadzu Spectrophotometer and Electronic spectra on UV-Vis 2500PC series spectrophotometerat IIT Patna.
Preparation of complex salts: -M{bis (glyoxalcarbohydrazone)}X2nH2O (M = CoII, NiII, PdII, ZnII and CdII, n = 0, 1 or 4, X =Cl‾ or 1/2 SO4-2). About 0.01 mol of carbohydrazide, 0.01molglyoxaldihydrate, and 0.005 mol metal chlorides were mixed in 100 ml water and 50 ml ethanol. The mixed reaction mixtures were refluxed on steam bath with constant stirring for 8–10 hours. The resulting solution was filtered and concentrated to 20-25 ml and pH was adjusted to 4-5 by adding a few drop of appropriate dilute acid. On cooling and standing the refluxateovernightin cold,microfine crystalline precipitate separated. The product were collected on Buckner funnel and washed with cold ethanol. The products were dried over CaCl2desiccators and analyzed.The complexsulphates were obtained when metal salt was taken as sulphate. The analytical results and molar electrical conductance value of products are mention in Table-A and B.
Results and discussion
The analytical results of macrocyclic complexes formed on condensation of glyoxaland carbohydrazide with CoII, NiII, PdII, ZnIIand CdIIsalts corresponds to composition M(glyoxalcarbohydrazone)2X2nH2O (M = CoII, NiII, PdII, ZnII and CdII, n = 0 forPdII,ZnIIand CdIIwhile 4 for CoII orNiIIand X = Cl‾ or 1/2 SO4-2). The complex salts are soluble in hot water or hot aqueous ethanol. The neutral complexes M(glyoxalcarbohydrazone)2X2nH2O dissolved in DMF and pyridine base on warming. The complexes are stable in air and aqueous medium. The electrical conductance value of complex salts in DMF occur in the range Ʌ∞ = 102–138 ohm-1mol-1cm2 indicating ionic nature of complexes7. As expected ZnII, CdII and PdII complexes are diamagnetic and CoII orNiII complexes are paramagnetic. The magnetic moment at room temperature of CoII complexes were found 4.92 and 5.01 for Co(glyoxalhydrazone)2X2nH2O (X = Cl‾ and 1/2 SO4-2). Which is similar to most of the spin free octahedral CoII complexes6–7. The magnetic moment value of NiII complexes (Table-B) occur in the range ofoctahedral environment of NiIIatom9.
The electronic absorption spectra of NiIIcomplexes displayed three distinct absorption band at 9680-9700, 16240-16360 and 24600-24840 cm-1 assignable to 3A2g →3T2g,→3T1g,→3T1g(P) transitions. The electronic absorptionspectra of aqueous ethanolic solution of CoIIcomplex salts displayed electronic transitions near 19250-19300 and 23400-23500 cm-1 as weak and broad bands due to d-d transitions. The electronic bands are assigned as 4T1g→4A2g and 4T1g→4T1g(P) transition as observed for octahedral CoIIatom7-8. The PdII complexes displayed a medium band at 425nm (23570 cm-1) attributed from 1A1g →1A2g transition in square planer field8-9. (Table-A)
The spectral parameters 10Dq, B and β for NiIIcomplexsalts [Ni(glyoxalcarbohydrazone)2(H2O)2]X2nH2O (X = Cl‾ or 1/2 SO4-2 and n = 2)were calculated using diagonal sum rule12and found to be 10Dq = 9680 cm-1, B =786 cm-1 and β = 0.75 forcomplex chlorides and 10Dq = 9700cm-1, B = 806cm-1 and β = 0.77forcomplexsulphates. The 20 – 25% reduction of β value indicated appreciable covalent character in complex10,12 [Ni(glyoxalcarbohydrazone)2(H2O)2]X2nH2O. The infrared spectra of complexes in KBr disc exhibit broad band at 3050-3400cm-1 attributed from ν(N-H),ν(C-H) and hydrogen bonded H2O and coordinated H2O of complexes molecule. The complexes shows a broad strong band at 1665-1652 cm-1 attributed from ν(C=O) of carbohydrazone (-NH-CO-NH-) group of cyclic ring 13,14. The medium band at 1592 cm-1 and 1540 cm-1are assigned to ν(C=N) and δ(N-H) of cyclic ring. The IR bands at 1430, 1332, 1256 and 1105 cm-1 are due to β(N-H), ν(C-C), ν(N-N) and ν(C-N) of cyclic ring system of the complex13. The complex sulphates display a strong a broad band at 1100-1120 cm-1and second weak band of 618 cm-1attributed from ionic sulphate group present in complex sulphates. A medium band at 836 and 735 cm-1 and weak bands at 635 and 506 cm-1areattributed to δ(N-H), δ(C-H) and deformation vibrations of ring system.A medium broad band located near 675-685 cm-1 is attributed to rocking vibration of coordinated water molecule13,14. Thus on the basis of above observationsmacrocycic structure is proposed to complex M(glyoxalcbhzH)2X2nH2O. The complexes of NiII and CoII possess octahedral structure with two coordinated H2O molecule while ZnII and CdII complexes are four coordinated tetrahedral but PdII complex [Pd(glyoxalcbhzH)2]Cl2 is squre planer.
Thermal stability: – The complexes are quite stable in air. The hydrated complexes [M(glyoxalcbhzH)2(H2O)2]SO42H2O gradually lose two water molecules on heating above 50oC and CdII or ZnII complexes become anhydrous below 90oC while NiIIand CoII complexes salts retain two H2Oand these are lost on heating above 160oC. The complexes decompose above 300oC to 380oCeither in metal oxide and complex sulphate. In case of sulphate salt the final residue of heated product was metal oxide obtained between 460oC to 530oC. The thermal stability was studied at heating rate 10oC per minute in static air. The decomposition of complex sulphate can be shown as follow on the basis of TGA studies.
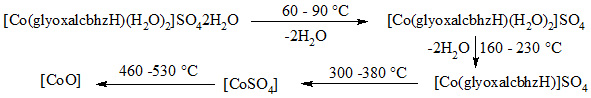
The probable structure on the basis of analytical results, electrical conductance data and their physical measurement are suggested for CoII, NiII,PdII,ZnII and CdII complexes as shown below.
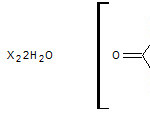 |
Scheme 1 Click here to View scheme |
[M = CoII or NiII and X = Cl‾ or 1/2 SO4-2]Tetrahedral for[M = ZnII or CdII]
Planer for PdII and n = 0 or 1 and X = Cl‾ or 1/2 SO4-2
Acknowledgement
One of the autherRakeshRoshan (UGC fellow) is thankful to the UGC, New Delhi for the financial assistance. We are also thankful to the authority of BIT Mesra, Ranchi, for recording TGA. IIT Patna for IR and UV-spectra.
Table 1: Analytical Table and physical data
| Complex Compound | Elemental analysis found (calc) | Electronic UV datacm-1 | ||
| % of M | % of N | % of anion | ||
| [Co{bis(glyoxalcbhzH)} (H2O)2]Cl22H2O | 13.3
(13.7) |
26.5
(26.3) |
16.4
(16.9) |
19000, 23000 cm-1 |
| [Ni{bis(glyoxalcbhzH)} (H2O)2]Cl22H2O | 13.4
(13.7) |
26.1
(26.3) |
16.6
(16.9) |
9680, 16200, 24600 cm-1, 10Dq = 9680 cm-1, B = 680 cm-1, β = 0.77 |
| [Pd{bis(glyoxalcbhzH)}]Cl2 | 26.1
(26.5) |
28.2
(27.9) |
23570 cm-1 | |
| [Zn{bis(glyoxalcbhzH)}]Cl2 | 17.7
(18.1) |
30.7
(31.1) |
19.6
(19.9) |
—– |
| [Cd{bis(glyoxalcbhzH)}]Cl2 | 27.3
(27.6) |
27.2
(27.5) |
17.1
(17.4) |
—– |
| [Zn{bis(glyoxalcbhzH)}]SO4H2O | 15.6
(16.0) |
27.5
(27.8) |
23.6
(23.8) |
—– |
| [Cd{bis(glyoxalcbhzH)}]SO4H2O | 25.2
(25.6) |
24.6
(24.9) |
20.8
(21.1) |
—– |
| [Ni{bis(glyoxalcbhzH)}(H2O)2]SO42H2O | 13.2
(12.9) |
25.2
(24.8) |
13.4
(13.1) |
9700, 16360 &14840 cm-110Dq=9700, B=806 cm-1, β=0.77 |
| [Co{bis(glyoxalcbhzH)}(H2O)2]SO42H2O | 13.4
(12.9) |
25.2
(24.9) |
13.3
(13.1) |
19250 & 23400 cm-1 |
Table 2
| Complex Compound | Color | µeff in BM R.T. | λαmol-1ohm-1cm2 |
| [Ni{bis(glyoxalcbhzH)}(H2O)2]Cl22H2O | Light green | 2.92 | 128 |
| [Co{bis(glyoxalcbhzH)}(H2O)2]Cl22H2O | Light pink | 4.92 | 138 |
| [Zn{bis(glyoxalcbhzH)}]Cl2 | Cream | Dia. | 122 |
| [Cd{bis(glyoxalcbhzH)}]Cl2 | Cream | Dia. | 131 |
| [Ni{bis(glyoxalcbhzH)}(H2O)2]SO42H2O | Bluish green | 3.01 | 120 |
| [Co{bis(glyoxalcbhzH)}(H2O)2]SO42H2O | Pinkish cream | 5.01 | 123 |
| [Zn{bis(glyoxalcbhzH)}]SO4H2O | Cream | Dia. | 128 |
| [Cd{bis(glyoxalcbhzH)}]SO4H2O | Cream | Dia. | 126 |
bis(glyoxalcbhzH) =bis (glyoxalcabohydrazone)
Reference
- Pederson C.J., J.Amer. Chem.Soc., 89: 7017 (1967).
- (A). Pederson C.J., Chem. Rev., 104: 2723 (2004). (B). Prasad R.N. and Sharma Monica., J. Indian. Chem. Soc., 83: 1260 (2006).C).Prasad R.N. and Mathur M., J. Indian. Chem. Soc., 80: 803 (2003).(D). Prasad R.N. and Sharma Nisha., J. Indian. Chem. Soc.,89: 29 (2012).
- Ohira A., Sakata M., Hirayama C. and Kunitake M., Org. Biomol. Chem., 1: 251 (2003).
- Uemura S., Sakata M., Taniguchi I., Hirayama C. and Kunitake M., Langmuir.,17: 5 (2001).
- Wang D., Xu O.M., Wan L.J., Wang C. and Bai C.L., Sur. Sci., 489L: 568 (2001).
- (A). Mishra L.K., and Jha Y., J. Indian. Chem. Soc., 76: 98-99 (1999).(B). Sinha B.K., Kant R. and Singh R., J. Indian. Chem. Soc., 76: 65-68 (1999).
- Geary W.J., Coord. Chem. Rev.,7: 81 (1971).
- Vogel A.I., “A Text Book of Quantitative Inorganic Analysis”, 4th Ed., Longmans Green, London. (1978).
- FiggisB.N. and Lewis J., Prog. Inorg. Chem.,6: 37 (1964).
- Lever A.B.P., “Inorganic Electronic Spectroscopy” Elsevier Amsterdam., Page 318, 324, 357. (1968).
- (A). Ballahausen C.J., “Introduction to ligand field theory” Mc.Graw Hill., NewYork. Page 256 (1962).(B). Jorgensen C.K., Adv. Chem. Phys., 5: 33 (1963).
- (A). Bostrup O. and Jorgensen C.K., Acta. Chem. Scand., 11: 1223 (1957).(B). Tanabe Y. and Sugano S., J. Phys. Soc. Japan., 9: 753 (1954).
- Belamy L.J., “The Infrared Spectra of Complex Molecule” 2nd Ed., Chapman and Hall. London. (1980)
- Feraro J.R., “Low Frequency Vibrations of Inorganic and Coordination Compound” Plenum., NewYork.(1971)

This work is licensed under a Creative Commons Attribution 4.0 International License.









