Extraction of Zn(II) Using magnetic chitosan nanoparticles grafted with β-cyclodextrin and determination by FAAS
Kamelia Karimnezhad1 and Ali Moghimi2*
1Faculty of science, Universiti Teknologi Malaysia, Skudai, Johor, 81310, Malaysia
2Department of Chemistry, Varamin(Pishva) branch, Islamic Azad University, Varamin Iran
DOI : http://dx.doi.org/10.13005/ojc/300112
Article Received on : December 12, 2013
Article Accepted on : January 19, 2014
Article Published : 27 Jan 2014
A novel and selective method for the fast determination of trace amounts of Zn(II) ions in water samples has been developed. The procedure is based on the selective formation of Zn(II) ions using magnetic chitosan nanoparticles grafted with β-cyclodextrin at different pH followed by elution with organic eluents and determination by atomic absorption spectrometry The preconcentration factor was 100 (1 ml elution volume) for a 100 ml sample volume. The limit of detection of the proposed method is 1.0 ng ml−1. The maximum sorption capacity of sorbent under optimum conditions has been found to be 5mg of Zn per gram of sorbent. The relative standard deviation under optimum conditions was 3.0% (n = 10). Accuracy and application of the method was estimated by using test samples of natural and synthetic water spiked with different amounts of Zn(II) ion.
KEYWORDS:Preconcentration; Zn (II); magnetic chitosan nanoparticles grafted with β-cyclodextrin; Solid phase extraction; Flame Atomic Absorption Spectrometry (FAAS)
Download this article as:| Copy the following to cite this article: Karimnezhad K, Moghimi A. Extraction of Zn(II) Using Magnetic Chitosan Nanoparticles Grafted with β-cyclodextrin and Determination by FAAS. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Karimnezhad K, Moghimi A. Extraction of Zn(II) Using Magnetic Chitosan Nanoparticles Grafted with β-cyclodextrin and Determination by FAAS. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=1980 |
Introduction
The direct determination of trace metals especially toxic metal ions such as Zn, tin, arsenic, lead, antimony and selenium from various samples requires mostly an initial and efficient pre-concentration step [1]. This pre-concentration is required to meet the detection limits as well as to determine the lower concentration levels of the analyte of interest [2]. This can be performed simply in many ways including liquid and solid phase extraction techniques [3,4]. The application of solid phase extraction technique for pre- concentration of trace metals from different samples results in several advantages such as the minimal waste generation, reduction of sample matrix effects as well as sorption of the target species on the solid surface in a more stable chemical form [5]. The normal and selective solid phase extractors are those derived from the immobilization of the organic compounds on the surface of solid supports which are mainly polyurethane foams [6], filter paper [7], cellulose [8] and ion exchange resins [9]. Silica gel, alumina, magnesia and zirconia are the major inorganic solid matrices used to immobilize the target organic modifiers on their surfaces [10] of which silica gel is the most widely used solid support due to the well documented thermal, chemical and mechanical stability properties compared to other organic and inorganic solid supports [11]. The surface of silica gel is characterized by the presence of silanol groups, which are known as weak ion exchangers, causing low interaction, binding and extraction of the target analytes [12]. For this reason, modification of the silica gel surface with certain functional groups has successfully been employed to produce the solid phase with certain selectivity characters [13]. Two approaches are known for loading the surface of solid phases with certain organic compounds and these are defined as the chemical immobilization which is based on chemical bond formation between the silica gel surface groups and those of the organic modifier, and the other approach is known as the physical adsorption in which direct adsorption of the organic modifier with the active silanol groups takes place [10].
Selective solid phase extractors and pre-concentrators are mainly based on impregnation of the solid surface with certain donor atoms such as oxygen, nitrogen and sulfur containing compounds [14–18]. The most successful selective solid phases for soft metal ions are sulfur- containing compounds, which are widely used in different analytical fields. Amongst these sulfur-containing compounds are dithiocarbamate derivatives for selective extraction of Co(II) [19,20] and pre-concentration of various cations [21,28-50] and 2- mercaptobenzothiazol-modified silica gel for on-line pre-concentration and separation of silver for atomic absorption spectrometric determinations [22]. Ammonium hexa-hydroazepin-1-dithiocarboxylate (HMDC)-loaded on silica gel as solid phase pre-concentration column for atomic absorption spectrometry (AAS) and inductively coupled plasma atomic emission spectrometry (ICP-AES) was reported [5]. Mercapto-modified silica gel phase was used in pre-concentration of some trace metals from seawater [23].
Sorption of Zn(II) by some sulfur containing complexing agents loaded on various solid supports [24] was also reported. 2-Amino-1- cyclopentene-1-dithiocaboxylic acid (ACDA) for the extraction of silver(I), Co(II) and palladium(II) [25], 2-[2-triethoxysilyl-ethylthio] aniline for the selective extraction and separation of palladium from other interfering metal ions [26] as well as thiosemicarbazide for sorption of different metal ions [27] and thioanilide loaded on silica gel for pre-concentration of palladium(II) from water [28,29] are also sulfur contaning silica gel phases. Most of the chitosan-based adsorbents were submicron to micron-sized and need large internal porosities to ensure adequate surface area for adsorption. However, the diffusion limitation within the particles led to the decreases in the adsorption rate and available capacity [30,31]. In this study, we report the synthesis of this new sorbent and its application as a selective sorbent for separation, preconcentration and determination of Zn2+ ions by AAS determination.
Experimental
Apparatus
Determination of Zn2+ contents in working samples were carried out by a Varian spectra A.200 model atomic absorption spectrometerequipped with a high intensity hallow cathode lamp(HI-HCl) according to the recommendations of the manufacturers. Separation of sorbent was assisted using a centrifuge (centurion scientific model: K 240R, West Sussex, U.K.). The pH measurements were carried out by an ATC pH meter (EDT instruments, GP 353). Fourier transform infrared (FTIR) spectroscopy measurements were performed by Bio-Rad model 400 using KBr as backgroundover the range of 4000–400 cm−1
Reagents and solutions
All reagents were of the highest purity available from Merck and were used as received. Analytical grade nitrate salts of litium, sodium, potassium, magnesium, calcium, strontium, barium, zinc, cadmium, lead, nickel, cobalt(II), and copper(II) were of the highest purity. Ultra pure organic solvents were obtained from E.Merck, Darmstat, Germany, and High Purity double distilled deionized water was used throughout the experiments.
The stock standard solution of Zn2+ was prepared by dissolving 0.1000g of the Zn powder in 10mL concentrated nitric acid and diluted to 1000mL with water in a calibrated flask. Working solutions were prepared by appropriate dilution of the stock solution. Glutaraldehyde and β -cyclodextrin were Aldrich products. N-(3-dimethylaminopropyl)-N´-ethylcarbodiimide (EDC) and 4-dimethylaminopyridine (DMAP) were Aladdin reagents. All other reagents used in this study were analytical grade, and distilled or double distilled water was usedin the preparation of all solutions.
Preparation and characterisation of the magnetic chitosan nanoparticles grafted with β-cyclodextrin
In this study ,a batch-wise process was employed for the extraction and preconcentration of Zn. Extraction was performed in test tubes containing Zn2+ in 10 ml acetate buffered solution (pH 3.0). Sixty milligrams magnetic chitosan nanoparticles grafted with β-cyclodextrin was added into the solution. After that, the mixture was shaken manually for an appropriate time to extract Zn completely from the solution.
The β-cyclodextrin -modified magnetic chitosan nanoparticles were prepared and modified with β-cyclodextrin to enhance the adsorption capacity for Zn. The adsorption behavior of the CMCN toward hydroquinol was studied. The equilibrium isotherms were determined and discussed. The bulk aqueous phase was removed with a pipette and any residual aqueous phase was easily decanted. The back extraction was performed using 1.0 ml of 1.0 mol l−1 methanol solution. The Zn concentration was determined by Flame Atomic Absorption Spectrometry (FAAS).The synthesis route of CMCN and its application are shown in Scheme 1B.[39]
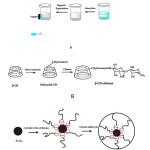 |
Scheme 1. Synthesis route of CMCN and its application for removal of hydroquinol with the help of an external magnetic field (A); schematic depiction of the formation of β -CD-chitosan particles (B); schematic depiction of the formation of magnetic cyclodextrin–chitosan (C). Click here to View figure |
Procedure
A batch-wise process was employed for the extraction and preconcentration of Zn. Extraction was performed in test tubes containing Zn2+ in 10 ml acetate buffered solution (pH 3.0). Sixty milligrams magnetic chitosan nanoparticles grafted with β-cyclodextrin was added into the solution. After that, the mixture was shaken manually for an appropriate time to extract Zn completely from the solution. Finally, test tubes were placed in centrifuge and separation of sorbent was achieved by centrifugation for 2.5 min at 3500 rpm. The bulk aqueous phase was removed with a pipette and any residual aqueous phase was easily decanted. The back extraction was performed using 1.0 ml of 1.0 mol l−1 methanol solution. The Zn concentration was determined by Flame Atomic Absorption Spectrometry (FAAS).
Results and discussion
Some preliminary experiments were carried out in order to investigate the extraction of Zn by the magnetic chitosan nanoparticles grafted with β-cyclodextrin from solution. The results showed that magnetic chitosan nanoparticles grafted with β-cyclodextrin can extract it quantitatively.
Characterization of magnetic particles and CMCN
Fig. 1 shows the SEM micrograph of magnetic particles and CMCN. The SEM analysis of the products provides information on the size and morphology of them. It can be seen from Fig. 1 that the magnetic particles have a particle shape with diameter distribution from 30 to 100 nm. Fig. 2 presents the FTIR spectra of chitosan (a), CMCN (b), and magnetic particles (Fe3O4) (c). As shown in Fig. 2, the adsorption and around 3420 cm−1, revealing the stretching vibration of N – H group bonded with O – H group in chitosan, and at 1661 cm−1 confirms the N- H scissoring from the primary amine, due to the free amino groups in the crosslinked chitosan. The IR spectrum of the cyclodextrin–chitosan demonstrates that β -CD is grafted onto the chitosan. The major peaks for magnetic modified chitosan in Fig. 2b can be assigned as follows: 3300 cm−1 (O- H stretching vibrations), 580 cm−1 (characteristic peaks of Fe3O4, as shown in Fig. 2c), 1650 cm−1 (C N stretching vibrations). The IR spectrum of the magnetic modified chitosan demonstrates that a layer of modified chitosan is formed on the surface of magnetite particles.[39]
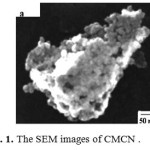 |
Fig. 1. The SEM images of CMCN Click here to View figure |
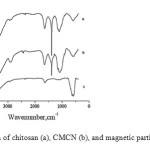 |
Fig. 2. IR spectra of chitosan (a), CMCN (b), and magnetic particles (Fe3O4) (c). Click here to View figure |
XRD patterns of CMCN (A) and pure Fe3O4 (B) are shown in Fig. 3, indicating the existence of iron oxide particles (Fe3O4), which has magnetic properties and can be used for the magnetic separation. The XRD analysis results of pure Fe3O4 and CMCN are mostly coincident.Six characteristic peaks for Fe3O4 (2Ɵ = 30.1, 35.5, 43.3, 53.4, 57.2 and 62.5), marked by their indices ((2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), and (4 4 0)), are observed in two samples. The surface areas of CMCN and cyclodextrin–chitosan were determined by nitrogen sorption measurements. The surface areas of CMCN and cyclodextrin–chitosan were 15.7 and 7.51 m2/g, respectively. The average pore diameter values of cyclodextrin–chitosan and CMCN were 2.89 and 3.16 nm. The relative adsorption performance of different adsorbent is highly dependent on the internal pore structure of each material. With the increase of pore diameter, more adsorbates are easier to be adsorbed. Therefore, crosslinking modification offers some attractive advantages. It is not only able to increase the surface area and average pore diameter, but also reinforce the chemical strength of adsorbents in acidic medium.[39]
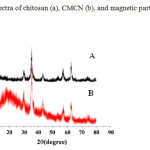 |
Fig. 3. XRD patterns of CMCN (A) and Fe3O4 (B). Click here to View figure |
These materials are potentially good candidates for the extraction of Zn(II) species from dilute aqueous medium because of the well-known tendency of amine derivatives to form stable complexes with Zn(II). Indeed, both aminopropyl-grafted silica gels[32] and carnosine functionalised silica[33] have been incorporated in carbon paste electrodes and successfully applied to electrochemical sensing of Zn(II) after preconcentration. In the present case, however, APS and Scar contain a great majority of their N centres in a protonated form. This is expected to limit their binding properties (decreasing stability of amine-Zn (II) complexes at pH below 7 [32]), thus requiring deprotonation of the material prior to use.
Effect of pH
Basically, the approach described above for Zn(II) detection at APS-MCPE should be applicable to any modified electrode system involving N-bearing ligands that would require a modulation of their properties by a pH change. To point out this generalisation aspect, we have revisited an earlier work dealing with the use of an amorphous silica sample functionalised with carnosine groups (Scar), which was applied as modifier of a carbon paste electrode (Scar-MCPE) to Zn(II) preconcentration and detection[33].
The effect of pH on the extraction of Zn2+ from water samples was studied in the pH range of 1.0–8.0. The higher pH values were not studied because magnetic chitosan nanoparticles grafted with β-cyclodextrin were not stable in alkali solutions due to the breaking of the Si–O–Si bonds by hydroxide ions attack54. pH of the solution was adjusted at the required value by adding 1.0 mol l−1 sodium hydroxide and/or 1.0 mol l−1 nitric acid. As can be seen in Fig. 4, extraction was nearly constant and quantitative in the pH range of 2–8.0. At lower pH (<2.5), the nitrogen atoms in magnetic chitosan nanoparticles grafted with β-cyclodextrin are protonated, so the stability of complex formation between the sorbent and Zn2+ is reduced. Therefore, the extraction of Zn decreased. Hence, pH of 3.0 was chosen as the optimum pH for extraction.
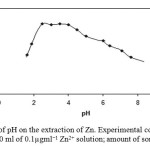 |
Fig. 4. Effect of pH on the extraction of Zn. Experimental conditions: source, 10 ml of 0.1µgml−1 Zn2+ solution; amount of sorbent, 50.0 mg Click here to View figure |
Choice of eluent
In order to choose the most effective eluent for desorbing Zn ion from the sorbent surface aliquots of 10 ml of 0.1µgml−1 Zn ion solution was contacted with 50.0 mg of magnetic chitosan nanoparticles grafted with β-cyclodextrin. A series of selected eluent solution such as nitric acid, formic acid, acetic acid, sodium thiosulfate, ethanol and methanol was used. A total of 10.0 ml of 0.1 mol l−1 of the above mentioned eluents were used for desorbing the adsorbed Zn ion. The amount of Zn ion back-extracted into the liquid phase by each eluent was measured using Flame Atomic Absorption Spectrometry (FAAS). Percent recoveries of Zn ion were calculated for each sample. The results (Fig. 5) showed that recovery was the best when methanol was used as eluent. Also, higher concentrations of hydrochloric and nitric acid (0.5 and 1.0 mol l−1) solutions were tested and the results showed the recovery of Zn was not quantitative. Therefore, methanol was selected as eluent.
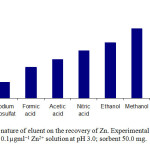 |
Fig. 5. Effect of nature of eluent on the recovery of Zn. Experimental conditions: source, 10 ml of 0.1µgml−1 Zn2+ solution at pH 3.0; sorbent 50.0 mg. Click here to View figure |
Effect of eluent concentration
The influence of the concentration of methanol on desorption of Zn ion was studied. For desorbing 1.0µg Zn ion, already adsorbed on 50.0 mg of sorbent, 1.0 ml of different concentration of eluent (methanol) have been used. At a concentration of more than 0.7 mol l−1, methanol desorbs (recovery of almost 100%) Zn ion completely from the sorbent surface. A concentration of 1.0 mol l−1 of methanol was selected for further studies.
Effect of the sample volume
In order to explore the possibility of concentrating low concentrations of Zn from large volumes, the maximum applicable volume must be determined. For this purpose, the effect of the sample solution volume on the recovery was studied by keeping the total amount of Zn2+ uptake constant (1.0µg). The quantitative recoveries were obtained for sample volume of ≤100 ml. Therefore, the concentration factor was 100 for Zn2+ since the final elution volume was 1.0 ml.
Effect of the amount of magnetic chitosan nanoparticles grafted with β-cyclodextrin
To test the effect of the amount of magnetic chitosan nanoparticles grafted with β-cyclodextrin on quantitative retention of analyte different amounts of sorbent (range from 2.0 to 200.0 mg) were added into the solution following the experimental method. The results showed that the extraction of Zn2+ was quantitative by using only 10.0 mg of magnetic chitosan nanoparticles grafted with β-cyclodextrin. Subsequent extraction experiments were carried out with 50.0 mg of magnetic chitosan nanoparticles grafted with β-cyclodextrin in order to achieve higher capacity and to account for other extractable species.
Adsorption capacity
The capacity of the sorbent is an important factor that determines how much sorbent is required to remove a specific amount of metal ions from the solution quantitatively. For investigation of adsorption isotherm of Zn ion, the same volumes of Zn ion solution with different concentrations of Zn ion were contacted with 0.1 g of sorbent in the batch mode. Then, the concentration of the remaining Zn in the solution was determined by Flame Atomic Absorption Spectrometry (FAAS). The adsorption isotherm that is the number of microgram absorbed per gram of adsorbent (Nf) versus the equilibrium concentration of cation (Cs) is shown in Fig. 6. According to these results, the maximum amount of Zn that can be sorbed by magnetic chitosan nanoparticles grafted with β-cyclodextrin was found to be 5mg g−1 at pH 3.0.
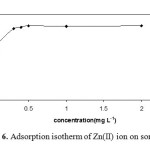 |
Fig. 6. Adsorption isotherm of Zn(II) ion on sorbent. Click here to View figure |
Effect of equilibrium time
In order to investigate the effect of shaking time on the extraction efficiency, extraction for a series of solutions containing 1.0µg Zn2+ were carried out. The results showed that the shaking time (from 20 to 350 s) has no effect on the extraction efficiency of Zn and the extraction was quantitative. Although the extraction process can be continued during the centrifugation, however, the results showed that extraction was quantitative and very fast in all cases. Thus, the mixtures have been shaken for 20 s to reach equilibrium in the subsequent experiments.
Effect of ionic strength
The influence of ionic strength on the extraction of Zn was studied in the potassium nitrate solution with various concentrations from 0.01 to 1.0 mol l−1. Results have shown that ionic strength has no considerable effect upon extraction efficiency up to 1.0 mol l−1 of KNO3. These observations showed the specific tendency of magnetic chitosan nanoparticles grafted with β-cyclodextrin for Zn2+ and the possibility of using this method for separation of Zn from highly saline solutions.
Effect of coexisting ions
The effects of common coexisting ions in natural water samples on the recovery of Zn were studied. In these experiments, 10 ml of solutions containing 0.1 µg l−1 of Zn and various amounts of interfering ions were treated according to the recommended procedure. An ion was considered to interfere when its presence produced a variation in the extraction recovery of sample more than ±5%. The results showed that, in excess of 10,000-fold Li+, K+, Na+, Ca2+, Mg2+, Ba2+, Sr2+ and 1000-fold Cl−, Br−, SO4 2−, Ag+, Cu2+, Cu2+, Ni2+, Zn2+, Mn2+, Pb2+, Al3+, Cr3+, Fe3+ and Hg2+ ions had no significant interferences in the extraction and determination of Zn. As can be seen, magnetic chitosan nanoparticles grafted with β-cyclodextrin has shown a high tolerance limit for alkali and alkaline earth metals. This is particularly useful for the analysis of Zn in natural water samples, for example, seawater, which contain large amounts of alkali and alkaline earth metal ions.
Reusability and stability of magnetic chitosan nanoparticles grafted with β-cyclodextrin
Reusability is one of the key parameters to assess the effectiveness of a sorbent. A series of sorption/desorption experiments were performed to understand the reusability of the synthesized magnetic chitosan nanoparticles grafted with β-cyclodextrin. After sorption, the sorbent was treated with 1.0 mol l−1 methanol to desorb Zn2+ and this sorption/desorption procedure was repeated five times. After each desorption step, the sorbent was washed with
doubly distilled water to remove methanol and condition sorbent. On storing for a year under dark and dry conditions, the stability of sorbent was excellent and adsorption capacity did not change significantly.
Analytical Performance
The limit of detection (LOD) and the limit of quantification (LOQ) were calculated as the amount of analyte necessary to yield a signal equal to three times (3σ) and ten times (10σ) the standard deviation of the blank signals, respectively. Using sample volume of 100 ml a LOD of 1.0µg l−1 and a LOQ of 1.2µg l−1 were obtained for the determination of Zn. Ten replicate extraction and measurement of 1.0µg of Zn2+ ion in 100 ml water solution gave a R.S.D. of 3.0%. Calibration graphwas obtained using preconcentration of 100 ml of standard solutions buffered at pH 3.0 with 50 mg of sorbent. For this purpose, standard solutions containing Zn ion in the range of 1–1000 µg l−1 were examined by the proposed procedure and it was observed that calibration curve were linear in this range. The regression equation was I = 0.0085C (µg l−1) + 0.0047 and the correlation coefficient was 0.9996.
Analysis of water samples
To assess the applicability of the method to real samples, it was applied to the extraction and determination of Zn from 100 ml of different water samples. Tap water(Tehran, taken after 10 min operation of the tap),rain water(Tehran, 26January, 2013), and Sea water(taken from Caspian sea, near the Mahmoud-Abad shore) samples were analyzed(Table 1). As can be seen from Table 1 the added Zn ions can be quantitatively recovered from the water samples used.
| Table 1 | |||
| Recovery of Zn(II) added to 100mL of different water samples (contaning 0.1M buffer acetic acid / acetate at pH= 3.0). | |||
| Sample |
Zn2+ spiked |
Zn2+ detected |
Recovery (%) |
|
(ng ml−1) |
(ng ml−1) |
||
| Sample 1a | 5.0 | 4.8 (2.0)b | 97.9 |
| 10.0 | 9.7 (2.5) | 98.6 | |
| Sample 2c | 5.0 | 4.4 (3.0) | 98.4 |
| 10.0 | 9.3 (3.0) | 98.3 | |
| Tap waterd | 0.0 | 2.5 (2.4) | 98.5 |
| 5.0 | 7.5 (2.9) | 97.5 | |
| 10.0 | 12.3 (2.4) | 98.3 | |
| Rain waterf | 0.0 | N.D. | — |
| 5.0 | 4.9 (2.3) | 98.4 | |
| 10.0 | 10.1 (2.6) | 98.6 | |
| Sea waterg | 0.0 | 14.0(2.0) | 99.3 |
| 5.0 | 18.9 (2.3) | 96 .9 | |
| 10.0 | 24.0 (3.0) | 98.1 | |
| a Hg2+,Cu2+,Zn2+,Fe3+,Ni2+,Cr3+ , 5000 ng ml−1 of each cation; K+ and Li+, 10,000 ng ml−1 of each. | |||
| b R.S.D of three replicate experiments. | |||
| c Hg2+,Cu2+,Zn2+,Fe3+,Ni2+,Cr3+ , 2500 ng ml−1 of each cation; K+ and Li+, 5000 ng ml−1 of each. | |||
| d From drinking water system of Tehran. | |||
| e Not detected. | |||
| f Tehran, 26january, 2013, Iran. | |||
| g Caspian sea water. | |||
Conclusion
Results presented in this work demonstrate well the tremendous possibilities offered by the solid phase extraction of trace amounts of Zn(II) in water samples using of magnetic chitosan nanoparticles grafted with β-cyclodextrin and its determination by FAAS. The method developed was simple, reliable, high capacity, good stability and fast adsorption and desorption kinetics for determining Zn in water. Also, the proposed method was free of interference compared to conventional procedures to determine Zn[34-38].The methode can be successfully applied to the separation and determination of Zn in binary mixtur.
Acknowledgements
The author wish to thank the Chemistry Department of Varamin-Pishva branch Islamic Azad University for financial support.
References
- D.E. Leyden, G.H. Luttrell, W.K. Nonidez, D.B. Werho, Anal. Chem. 48 (1976) 67.
- J.S. Jones, D.E. Harrington, B.A. Leone, W.R. Bramdstedt, Atom. Spectrosc. 4 (1983) 49.
- D.C. Nambiar, N.N. Patil, V.M. Shinde, Fresenius J. Anal. Chem. 360 (1998) 205.
- C. Caroli, A. Alimanti, F. Petrucci, Zs. Horvath, Anal. Chim. Acta. 248 (1991) 241.
- A. Alexandrova, S. Arpadjan, Analyst 118 (1993) 1309.
- S. Arpadjan, L. Vuchkova, E. Kostadinova, Analyst 122 (1997) 243.
- D.E. Leyden, G.H. Luttrell, Anal. Chim. 47 (1975) 1612.
- M.C. Gennaro, C. Baiocchi, E. Campi, E. Mentasti, R. Aruga, Anal. Chim. Acta 151 (1983) 339.
- M. Grote, A. Kettrup, Anal. Chim. Acta 175 (1985) 239.
- K. Unger, Porous Silica, Elsevier, Amsterdam, 1979.
- S.P. Boudreau, W.T. Cooper, Anal. Chem. 61 (1989) 41.
- R.J. Kvitek, J.F. Evans, P.W. Carr, Anal. Chim. Acta 144 (1982) 93.
- M.L. Bruening, D.M. Mitchell, J.S. Bradshaw, R.M. Izatt, R.L. Bruening, Anal. Chem. 63 (1991) 21.
- M.E. Mahmoud, Talanta 45 (1997) 309.
- M.E. Mahmoud, E.M. Soliman, Talata 44 (1997) 15.
- M.E. Mahmoud, E.M. Soliman, Talanta 44 (1997) 1063.
- A. Tong, Y. Akama, S. Tanaka, Anal. Chim. Acta 230 (1990) 179.
- V. Dadler, L.F. Lindoy, D. Sallin, C.W. Schlaepfer, Aust. J. Chem. 40 (1987) 1557.
- M.E. Mahmoud, in: Proceeding of the 25th FACSS Conference, Austin, TX, USA, 11–15 October, 1998.
- M.E. Mahmoud, Anal. Chim. Acta 398 (1999) 297.
- D.E. Leyden, G.H. Luttrell, A.E. Sloan, N.J. DeAngelis, Anal. Chim. Acta 84 (1976) 97.
- P. Qiaosheng, S. Qiaoyu, H. Zhide, S. Zhixing, Analyst 123 (2013) 239.
- A.Moghimi ;N. TAJODINI Asian Journal of Chemistry Vol. 22, No. 5 (2010), 3325-3334.
- N. TAJODINI ; .Moghimi Asian Journal of Chemistry Vol. 22, No. 7 (2010), 4994-5000.
- A.Moghimi, M.S.Tehrani, S.Waqif Husain, Material Science Research India 3(1a) (2006)27.
- M.S.Tehrani, A.Moghimi, S.Waqif Husain, Material Science Research India 3(2)(2005)135.
- M.E. Campderros, A. Acosta, J. Marchese, Talanta 47(2012)19.
- I.Narin, M. Soylak, L.Elic, M.Dogan, Talanta 52(2011)1041.
- T.Z. Zhou, D.Y. Qi, C.P. Zhang, Acta Chim. Sin. 41(1983) 237.
- L.M. Zhou, J.Y. Jin, Z.R. Liu, X.Z. Liang, C. Shang, J. Hazard. Mater. 185 (2011) 1045–1052.
- L. Lian, L. Guo, C. Guo, J. Hazard. Mater. 161 (2009) 126–131. 32 . Shin D. H., Ko Y. G., Choi U. S., Kim W. N., Ind. Eng. Res.2004, 43, 2060.
- Etienne, M. ; Bessiere, J.; Walcarius, A.; Sensor Actuator B,B76 (2001) 531.
- P.Nayebi,; A.MOGHIMI, Oriental Journal of Chemistry 22(3) (2006) 507.
- Choi,Y.S.;Choi,H.S.Bull.Korean Chem. Soc.24(2013)222.
- Matoso,E.;Kubota,L.T.;Cadore,S. Talanta 60(2013)1105.
- Purachat, B.; Liawruangrath,S.;Sooksamiti,P.;Rattanaphani,S.;Buddhasukh, D.Anal.Sci. 17(2001)443.
- Ensafi,A.A.;Abbasi,S.;Rahimi Mansour,H.;Mohammad pour Baltork, I.Anal.Sci. 17(2001)609.
- Saber Tehrani,M.; Rastegar,F.; Parchehbaf, A.;Rezvani,Z.;Chinese Journal of Chemistry 23(2005)1437.
- L. Fan, M. Li , Z. Lv, M. Sun, C. Luo, F. Lu, H. Qiu Colloids and Surfaces B: Biointerfaces 95 (2012) 42– 49.

This work is licensed under a Creative Commons Attribution 4.0 International License.









