Soft Synthesis and Nano -Structural Features of Highly Crystalline Asprin An AFM-Investigations
Morsy M.A.Sekkina1 , Khaled M. Elsabawy1,3* and A. El-Maghraby2,3
1Materials Science Unit , Chemistry Department ,Faculty of Science, Tanta University-31725-Tanta –Egypt
2Ceramic Department, National Research Center, Dokki, Tahrir st. ,Egypt
3 Department of Chemistry, Faculty of Science, Taif University, 888- Taif, Kingdom of Saudi Arabia
DOI : http://dx.doi.org/10.13005/ojc/290417
Article Received on :
Article Accepted on :
Article Published : 15 Jan 2014
The present investigations introduce new trend of applying AFM-microscopy to visualize a real 3D-imaging of sample’s surface topography .High resolution AFM-investigations indicated that crystalline asprin has regular arrays of atomic arrangement with no violation in the bulk of asprin .TM deflection AFM- gave us good approximation to the diffusion of grain throughout the surface topology of investigated asprin . The AFM-deflection centers imaging indicated that the numbers of grains distribute in circular arrangements on the surface and material bulk .3D-visualized imaging introduce precise determination of exposure surface area interacting with dissolving agent .
KEYWORDS:AFM-microscopy ; Crystalline ;Grain ; Nano-structural Features ;Deflection centers.
Download this article as:| Copy the following to cite this article: Sekkina M. M. A, Elsabawy K. M, El-Maghraby A. Soft Synthesis and Nano -Structural Features of Highly Crystalline Asprin An AFM-Investigations. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Sekkina M. M. A, Elsabawy K. M, El-Maghraby A. Soft Synthesis and Nano -Structural Features of Highly Crystalline Asprin An AFM-Investigations. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1669 |
Introduction:
Aspirin has a direct irritant effect on gastric mucosa due to inhibition of prostaglandins and prostacyclin and thus causes ulceration, epigastric distress and haemorrhage. So as to reduce the side-effects the controlled release formulation of aspirin has to be prepared [1,2] . Chemically, aspirin is degraded by water to salicylic acid and acetic acid. Drugs in the solid state can have significant influences on a variety of physical and chemical properties [3] and it is essential to characterize the effect of moisture on these individual components. [4]Direct compression is the most efficient process used in tablet manufacturing but it requires different properties of powder such as good flowability, good compressibility, and bulk density. Many of the crystals do not exhibit these properties; hence it is necessary to improve these properties. As, Aspirin is having poor flowability and compressibility, it is necessary to increase the flowability and compressibility of Aspirin also it is moisture sensitive drug, hence there is need to avoid these major problems [5, 6]
All the problems associated with Aspirin could be overcome by the technique known as spherical crystallization, is a novel particle engineering technique by which crystallization and agglomeration can be carried out simultaneously in one step to transform drug crystals directly into a compacted spherical form and direct compression is possible. Spherical crystallization has been developed by Yoshiaki
Kawashima and co-workers as a novel particulate design technique to improve processibility such as mixing, filling, tableting characteristics and dissolution rate of pharmaceuticals.[7]
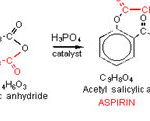
The synthesis of asprin involves the reaction of salicylic acid and acetic anhydride in the presence of phosphoric acid, H3PO4 as catalyst [8,9].
 |
Click here to View figure |
The isolation and purification of asprin , once the aspirin is prepared it must be isolated from the reaction solution and purified.
The acetic acid and phosphoric acid are water soluble and can be removed by washing the aspirin with chilled water. Salicylic acid is only slightly soluble in water and is not completely removed in the washing step. Final purification is accomplished by the process of recrystallization. The impure aspirin is dissolved in warm ethanol. The solution is then cooled slowly, and the aspirin crystallizes out of solution leaving the salicylic acid and other impurities behind.
Experimental :
Synthesis of Crystalline Asprin :
Using an electronic pan balance, weigh out 2.0 g of salicylic acid and transfer it to a cleanand dry 125 mL Erlenmeyer flask. 4.0 mL of acetic was added anhydride to the flask and gently swirl the flask for a minute. Carefully 3-5 drops of concentrated phosphoric acid was added . The Erlenmeyer flask was placed in a hot water bath in the hood and let it heat for 5 minutes while swirling the flask occasionally. During this time period all of the salicylic acid should dissolve. Add 30 mL of distilled water to the flask, swirl it to mix all the reagents then let it sit in the water bath for 1 minute. The water you added will convert any unreacted acetic anhydride to acetic acid. Remove the flask from the hot water bath and let it
cool to room temperature. The mixture should become gummy then a clump of solid should crystallize out. Wash the flask and the collected aspirin in the funnel with two 10 mL portions of ice cold distilled water. Dry the solid crystal by pulling air through the funnel for five more minutes.
Ultra-Crystallization of Asprin :
Three equivalent weights of highly pure asprin powders (each of 0.4 gm ) were dissolved in 30 ml of warm ethanol with supporting ultrasonic instrument . The re-crystallization process was performed using gently microwave assist to avoid any traces from applied solvent . The highly pure crystals were dried in oven the forwarded for structural investigations
Structural measurements :
The X-ray diffraction (XRD): Measurements were carried out at room temperature on the fine ground samples using Cu-Kα radiation source ,Ni-filter and a computerized STOE diffractometer/Germany with two theta step scan technique. Rietveld and indexing of structure were made via Fullprof package and Gesas program.
Scannig electron microscopy (SEM): measurements were carried out along ab-plane using a small pieces of the prepared samples by using a computerized SEM camera with elemental analyzer unit Shimadzu (Japan).Atomic force microscopy (AFM): High-resolution Atomic Force microscopy (AFM) is used for testing morphological features and topological map (Veeco-di Innova Model-2009-AFM-USA).The applied mode was tapping non-contacting mode. For accurate mapping of the surface topology AFM-raw data were forwarded to the Origin-Lab version 6-USA program to visualize more accurate three dimension surface of the sample under investigation. This process is new trend to get high resolution 3D-mapped surface for very small area.
RESULTS &DISCUSIONS
The crystalline asprin sample was structurally and spectrophotometrically examined by both of X-ray diffraction as shown in Fig.1 and infrared spectroscopy respectively and well established as crystalline asprin which forwarded to AFM-and SEM to investigate nano-structural features.
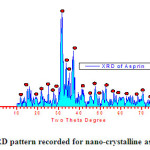 |
Fig.1 : XRD pattern recorded for nano-crystalline asprin Click here to View fiure |
 |
Fig.2 SE-micrograph captured for crystalline asprin Click here to View figure |
The analysis of SE-micrograph recorded for crystalline asprin indicated that the grain size ranged in between 170-200 nm and no in-homogeneities observed on the grain boundaries or in between grain which refer to the quality of synthesis applying solution route[8-9] .The balck arrows in Fig.2 display moderate to large size gains while red arrows are foe small size gains .
The average of grain size was estimated from XRD data pattern applying Scherrer’s formula [10] and found to be 196 nm . This result is fitted with grain size estimated from SEM examinations .
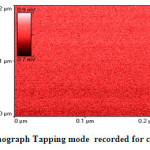 |
Fig.3a AFM-Nanograph Tapping mode recorded for crystalline asprin Click here to View figure |
Fig.3a shows AFM-nano-graph captured for crystalline asprin applying tapping mode technique for 0.04μm2 scanned area . The analysis of AFM-nano-graph of crystalline asprin indicated that the average grain size was found 167 nm which is consistent with those calculated from scanning electron micrograph image that reflect the quality of synthesized asprin by using solution route technique .
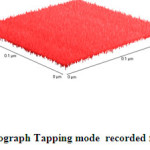 |
Fig.3b 3D-AFM-Nanograph Tapping mode recorded for crystalline asprin Click here to View figure |
As clear in Fig.3b which describe 3D-topography of crystalline asprin’s surface no abnormal heights present on the whole scanned area 0.2×0.2 μm which reflects the quality of solution route synthesis as preparation technique for crystalline asprin even at very small area .These results indicate that the homogeneity degree are maximum with very small ratio of impurity phases as confirmed in XRD pattern recorded for crystalline asprin see Fig.1 .
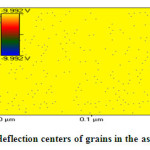 |
Fig.3c TM-deflection centers of grains in the asprin topology Click here to View figure |
Fig.3c shows deflection centers distribution of grain through scanned surface area 0.04 μm2 .As clear the in figure black dots refer to grain orientation on the surface which regularly distributed in circular arrangement reflect degree of homogeneity on the material surface .Only very few numbers of grains distributed irregularly due to jamming of population during re-crystallization process which affected by heating rate and solvent applied in crystallization process [ 11-17 ] .The connections of deflection dots could be benefit to understand both of micro-structural features and conduction mechanisms within material surface’s .
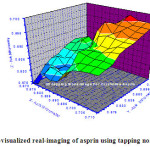 |
Fig.4 : 3D-AFM-visualized real-imaging of asprin using tapping non-contact mode Click here to View figure |
Fig.4 shows 3D-visualized image for crystalline asprin with maximum heights ranged in between 0.69-0.70 μm which represented by orange-red color with ratio ~ 7% of total scanned area .The minimum depth with blue gradient color is nearly 18% recording 0.677-0.680 μm.
These details of surface topography enhance us to calculate and estimate accurately the surface exposure area of the whole scanned area which is responsible fore solubility interactions with any dissolving solvent .
Conclusions :
The conclusive remarks can be summarized in the following points
– The topology of asprin surface acts important role in its solubility rate .
– Crystalline asprin have 3D-regular array net of asprin decreasing steric hiderence problem as organic molecule .
– 3D-AFM-visualized imaging introduced sharp informative conclusions about the inernal layered structure of nano-array of crystalline asprin within narrow scanned area reach to 0.01 μm2 .
References:
- Byrd, H.; O’Donnell, Stephen E., J. Chem. Educ. 80, 174( 2003).
- Olmsted, John A. III; J. Chem. Educ. 75, 1261.(1998).
- Analgesics K., Anti-inflammatory Drugs and Antipyretics. In: Martindale. The Complete Drug Reference, 32nd Raderick PJ, Wilkes HG, Meade TW. Br J Clin Pharmacol, 35: 219–226. (1993).
- Parfitt ed., London; Pharmaceutical Press: 2–12 (1999).
- Claes A., Reck, George.Int J Pharmaceutics, 62,87-95(1990), Anuradha,et al. Int J Pharm , 3(1), 108-115 (2013).
- Dubinin M.M. Investigations of equilibrium and kinetics of adsorption of gases on Zeolites, Washington; American Chemical Society Publications: pp. 1 – 15 (1977) .
- Rasmuson C., Katta J. Int J Pharm, 348, 61-69( 2008).
- Shangraw R.F. Direct Compression Tableting: Encyclopedia of Pharmaceutical Technology, NewYork; Marcel Dekker: pp. 85-160 (1988).
- Kawashima Y., Okumura M, Takenaka H. Powder Technol,39,41–47(1984).
- Hileman G.A., Goskonda S.R., Spalitto A.J., Drug Dev Ind Pharm, 19(4): 483–491(1993).
- Chariot M., Frances J., Lewis G.A., Mathieu D., Phan Tan Luu R., Stevens HNE. Drug Dev Ind. Pharm, 13 (9–11), 1639–1649(1987).
- Bodea A., Leucuta S.E. Int J Pharm, 154(1),49–57(1997).
- Nayak A.U., Mutalik S., Reddy M.S., Averineni K.R., Kushtagi P., Udupa N. Eur J Pharm Biopharm, 70, 674–683( 2008).
- Pawar P.H., Pawar AP, Madhik KR, Pawar AP, Paradkar AR. Indian J Pharm Sci, 60, 24-28(1998).
- Shaikh A.A., Pawar YD, Kumbhar ST. Int J Pharm Sci Res, 3(5), 1411-1414(2012).
- Conners K.A., Amidon GL, Stella VJ. Chemical Stability of Pharmaceuticals, New York Wiley-Interscience: (1986).
- Goyal N., Sharma N., Sharma P. Der Pharmacia Lettre,; 2(4), 246-254(2010).

This work is licensed under a Creative Commons Attribution 4.0 International License.









