A Stability Indicating RP-HPLC for the Simultaneous Estimation of Hydrochlorothiazide, Amlodipine Besylate and Telemisartan in Bulk and Pharmaceutical Dosage form
K. Kalyani1*, V. Anuradha2, S. Vidyadhara3, R. L. C. Sasidhar3 and T. N. V. Ganesh Kumar3
1Department of Chemistry, R.V.R and J.C College of Engineering, Guntur, A. P., India.
2Vignan’s Nirula Institute of Technology and Science, Guntur, A.P., India
3Chebrolu Hanumaiah Institute of Pharmaceutical Sciences, Guntur, A.P., India.
Corresponding Author's E-mail: kk.chem445@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320340
Article Received on : March 11, 2016
Article Accepted on : April 17, 2016
Article Published : 23 May 2016
A stability indicating RP-HPLC method was developed for the simultaneous estimation of the anti hypertensive drugs Hydrochlorothiazide, Amlodipine Besylate and Telemisartan. These drugs were subjected to stress studies under different conditions as per ICH guidelines. The separations were carried out using C18 reverse phase column (Agilent ODS UG 5 column, 250mm x 4.5mm,5µm) employing Acetonitrile and Acetate buffer (60:40 v/v) as mobile phase and pH adjusted to 5 at flow rate of 1ml/min was used for separation, deteced at 333 nm. The drugs were exposed to acidic, alkaline, oxidative, thermal and photolytic conditions and the stressed samples were analyzed by the proposed method. Degradation studies showed that all the three drugs were degraded under oxidative, thermal and photolytic conditions, negligible degradation observed under acidic, alkaline conditions. Analytical validation parameters such as specificity, linearity, accuracy, precision, Ruggedness and Robustness were determined and relative standard deviation of all the parameters were found to be less than 2%. Hence this method was found to be stable indicator that can be used for the routine analysis of these drugs in the bulk and combined tablet dosage form.
KEYWORDS:Hydrochlorothiazide;Amlodipine Besylate;Telemisartan;RP-HPLC;Stress studies
Download this article as:| Copy the following to cite this article: Kalyani K, Anuradha V, Vidyadhara S, Sasidhar R. L. C, Kumar T. N. V. G. A Stability Indicating RP-HPLC for The Simultaneous Estimation of Hydrochlorothiazide, Amlodipine Besylate and Telemisartan in Bulk and Pharmaceutical Dosage form. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Kalyani K, Anuradha V, Vidyadhara S, Sasidhar R. L. C, Kumar T. N. V. G. A Stability Indicating RP-HPLC for The Simultaneous Estimation of Hydrochlorothiazide, Amlodipine Besylate and Telemisartan in Bulk and Pharmaceutical Dosage form. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=16384 |
Introduction
Hydrochlorothiazide – C7H8ClN3O4S2(fig.1a) (HCT) chemically known as 6-chloro-3, 4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulphonamide, 1-dioxide.It is a first-line diuretic drug. Amlodipine besylate –C20H25ClN2O5 (fig.1b) (AMB) chemically known as (RS)-3-ethyl 5-methyl 2-[(2-aminoethoxy) methyl]-4-(2-chlorophenyl)-6-methyl-1, 4-dihydropyridine-3, 5-dicarboxylate mono benzene sulfonate. It is used to treat pressure andis a potent dihydro calcium channel blocker. Telmisartan C33H30N4O2 (fig.1c) (TEL) chemically 4′-[(1, 4’-dimethyl-2′-propyl [2, 6’-bi-1H-benzimidazol] – 1’-yl) methyl]-[1, 1’-biphenyl]-2- carboxylic acid. It is also used to treat pressure and an angiotensin converting enzyme inhibitor and angiotensin – II type I receptor blocker1,3. Literature survey reveals that there weremany analytical methods for all three drugs alone or in combination with other drugs including spectroscopic and chromatographic methods are reported4-10, but there is no method established for the stability indicating RP-HPLC under stress for this combination. The present work describes the development of stability indicating RP-HPLC method, which can quantify these components simultaneously from a combined dosage form. The present RP-HPLC method was validated 11,12 and applied under stressed conditions according to International conference on harmonization (ICH) guidelines. ICH has made the mandatory need of developing stability indicating assay methods for every drug candidates. There stability indicating assay methods helps in establishing the inherent stability of the drug which provides assurance on detection changes in identity, purity and potency of the product on exposure to various conditions.In the present study Hydrochlorothiazide, Amlodipine Besylate and Telemisartan is exposed to a variety of stress like acidic, basic, thermal, photolytic and oxidative stress conditions13. According to ICH guidelines the stress testing of the drug substances helps in identifying the likely degradation products, which in turn can help in establishing the degradation pathways and the intrinsic stability of the molecule. The aim of the present work was to develop stability indicating method for determination of all the three drugs in presence of its degradation products.
 |
Figure 1: Hydrochlorothiazide, figure 2: Amlodipine Besylate figure 3: Telmisartan Click here to View figure |
Experimental
Instrumentation
Analysis was performed with an Agilent ODS UG 5 column, 250mm x 4.5mm with C18 column of injection capacity of 20 μL. The mobile phase was a 60:40% (v/ v) mixture of Acetonitrile: Acetate Buffer (50 mM, pH 5 ± 0.1, adjusted with orthophosphoric acid). The flow rate was 1.0 mL min−1 and the run time was 15 min. Before analysis both the mobile phase and sample solutions were degassed by the use of a sonicator (1.5LH Ultrasonic bath sonicator) and filtered through a 0.45 µ membrane filter paper. The identity of the compounds was established by comparing the retention times of compounds in the sample solution with those in standard solutions. Chromatography was performed in an ambient temperature maintained at 20 ± 1°C. The UV spectrum of HCT, AMB and TEL for selecting the working wavelength of detection was taken using a LAB INDIA double beam UV – Visible spectrophotometer with pair of 10 mm matched quartz cells.
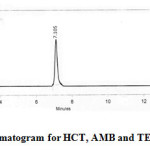 |
Figure 2: Optimized chromatogram for HCT, AMB and TEL Click here to View figure |
Reagent and chemicals
Pharmaceutically pure sample of HCT, AMB and TEL were obtained as a gift samples from Mylan laboratories. All solvents were of AR grade obtained from S.D. Fine chem. Limited, Mumbai. A combination of HCT (12.5 mg), AMB (5 mg), and TEL (40 mg) in tablet formulation was procured from Indian market TELVAS*-3D, Aristo pharmaceutical Pvt. Ltd, India.
Preparation of Stock Solution of HCT, AMB and TEL
About 50mg of HCT, AMB and TEL were accurately weighed and transferred in to 50 mL volumetric flasks separately. It was dissolved in methanol and the solution was made up to the volume with same solvent.
Method of Validation
The linearity of the prepared method was studied by injecting different concentrations of the HCT, AMB and TEL prepared in the mobile phase in the range of 20-100µg/mL and the sample solutions were injected into HPLC system and peak areas were recorded. Precision of the method was demonstrated by interday and intraday variation studies. In the intraday studies analysis of 100% concentration of six replicate injections were performed in a day. In the interday variations studies the analysis of the drug was repeated on three consecutive days. Accuracy of the method was determined by recovery experiments. As known quantity of pure drug was added to the pre analysed sample and the contents were re analysed by the proposed method and percent of recovery was reported.
Table 1: Results from study of linearity
|
Parameters |
HCT* |
AMB* |
TEL* |
|
Detection wavelength |
333nm |
||
|
Beer’s law limit (µg mL-1) |
20-100 |
20-100 |
20-100 |
|
Correlation Coefficient (r) |
0.999 |
0.999 |
0.999 |
|
Regression equation (y= mx+c) |
Y= 14245x + 40813 |
Y= 10121x + 15999 |
Y=11079 x -25536 |
|
Slope |
14245 |
10121 |
11079 |
|
Intercept (a) |
40813 |
15999 |
25536 |
|
LOD (µg mL-1) |
5.2 |
9.45 |
7.60 |
|
LOQ (µg mL-1) |
17.16 |
31.18 |
25.08 |
*Mean of six determinations.
The limit of detection and limit of quantitation were calculated from the slope of calibration curve. Robustness of the method was determined by carrying out the analysis by altering the conditions of mobile phase composition and flow rate and the effect on the area of peak of intercept and retention times were determined.
Assay of marketed formulation
The contents of twenty commercial tablets were weighed and their mean mass was determined. After grinding the tablets into a fine powder in a glass mortar, an accurately weighed quantity of the tablet powder equivalent to 40 mg of TEL was quantitatively transferred into a 50 ml volumetric flask. The contents were sonicated for 15 min, to ensure the complete solubility of the drug. The solution was made up to the mark with the solvent and filtered through a 0.45 µ membrane filter paper. From the clear solution, further dilutions were made by diluting 1.0 mL into 10 mL with mobile phase; to obtain 100µg mL-1 of TEL and 31.25µg mL-1 of HCT and 12.5 µg mL– 1 of AMB. Each sample solution was injected and the peak areas were measured for the determination of HCT, AMB and TEL in tablet formulation (table-2)
Table 2: Results from assay of tablet formulation
|
Amount Present in (mg/tab) |
Amount Obtained in (mg/tab) |
Label Claim %w/w |
||||||
| HCZ | AMB | TEL | HCZ | AMB | TEL | HCZ | AMB | TEL |
| 12.5 | 5 | 40 | 12.48 | 4.99 | 40.05 | 99.84 | 99.8 | 100.12 |
Degradation Studies
Forced degradation or stress test is undertaken to demonstrate specificity, when developing stability- indicating methods, particularly when little information is available about potential degradation products. These studies also provide information about the degradation pathways and degradation products that could form during storage. Forced degradation studies may help facilitate pharmaceutical development as well in areas such as formulation development manufacturing and packaging in which knowledge of chemical behaviour can be used improve a drug product 14- 16.
Stress Degradation by Hydrolysis under Acidic Conditions
For stability studies 2mL of sample was taken from the HCT, AMB and TEL injection solution into a 100mL volumetric flask and diluted up to the mark using mobile phase.
Acid hydrolysis of the drugs were carried out in presence of 1.5N HCl. The drug solution was refluxed with 1.5 N HCl for 2hrs. After exposure for the required duration of time the stressed samples were sonicated and diluted to a concentration of 100µgm/mL with mobile phase and then diluted to 10µg/mL and was injected into the HPLC system after filtration.
Stress Degradation by Hydrolysis under Alkaline Conditions
The alkali hydrolysis of the drug was carried out in 0.1N NaOH and refluxing the solution for 8hrs. After exposure the stressed samples were suitably diluted with mobile phase and then injected into HPLC system.
Oxidative Stress
The drug solution was prepared by dissolving 5mL of HCT, AMB and TEL solution in 10mL of 1% hydrogen peroxide and refluxing the solution at 300C for 24 hours. After exposure the samples were diluted, filtered and injected into the HPLC system.
Thermal degradation
HCT, AMB and TEL sample solutions was taken in a volumetric flask and kept in an oven maintained at 700 C temperature for 24 hours. Then the sample solution was dissolved and diluted in mobile phase. From this appropriate dilution was made mobile phase and injected in chromatographic system.
Photolytic degradation
5mL of HCT, AMB and TEL samples solution was exposed to ultraviolet lamp in a photostability chamber for 2 hrs. After exposure the stressed sample solution was suitably diluted with mobile phase and injected under stabilized chromatographic conditions.
Results and Discussion
The present study was aimed to develop a simultaneous validated stability indicating RP- HPLC method for the estimation of anti-hypertensive drugs Hydrochlorothiazide , Amlodipine besylate and Telemisartan. After several trails with different mobile phase compositions, a 60:40 (v/ v) mixture of Acetonitrile: Acetate Buffer(50 mM, pH 5 ± 0.1, adjusted with orthophosphoric acid) was found to be good. This ratio was optimised as mobile phase for the simultaneous estimation of all the three drugs.
The data obtained from the calibration experiments when subjected to linear regression analysis showed a linear relationship in peak areas and concentration in the range of 20- 100µg/mL for each drug. The developed method was found to be precise as the %RSD values for intra-day and inter-day precision studies were found to be less than 2%. The recovery studies obtained at each added concentration indicates the accuracy of this method. The limit of detection of HCT, AMB and TELwas found to be 5.2, 9.45, 7.6 µg/mL and limit of quantification was 17.16, 31.18, 25.08 µg/mL respectively thus indicating the sensitivity of the method (table 1). While evaluating the robustness, the peak areas of all the three dugs were found to vary within +0.009%, whereas the retention was found to vary in the range. The method was robust as the peak areas and retention time were not significantly affected after varying the parameters like flow rate and wave length. The assay of commercial tablets was established with present chromatographic condition developed and it was found to be more accurate and reliable. The percentage label claim present in tablet formulation TELVAS*-3D was found to be 99.84, 99.8, 100.12 for HCT, AMB and TEL, respectively (Table 2). The chromatogram for the analysis of formulation is shown in Figure 3.
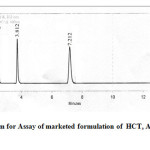 |
Figure 3: Chromatogram for Assay of marketed formulation of HCT, AMB and TELClick here to View figure |
The drugs were exposed to acidic, base, oxidative, thermal and photolytic conditions and the stressed samples were analysed by the proposed method. Degradation studies showed that all the three drugs were degraded under oxidative, thermal and photolytic conditions. The drug peak areas decreased sufficiently with drastic change in the Rt values. Two new peaks were observed in chromatograms of acid and alkaline as when compared to the standard, which progressed without any change in Rt. The chromatograms of the stability studies were shown in the figures 4-7.
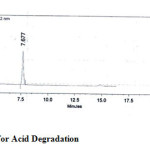 |
Figure 4: Chromatogram for Acid Degradation Click here to View figure |
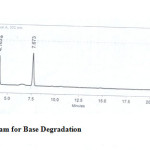 |
Figure 5: Chromatogram for Base Degradation Click here to View figure |
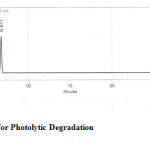 |
Figure 6: Chromatogram for Photolytic Degradation Click here to View figure |
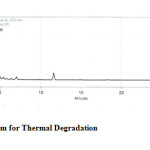 |
Figure 7: Chromatogram for Thermal Degradation Click here to View figure |
This present work on the three drugs Viz., HCT, AMB and TEL shows maximum degradation in photolytic, thermal and oxidative conditions whereas relative stability was noticed when they were exposed to acidic and alkaline conditions. Thus the developed RP-HPLC method was found to be simple, rapid, sensitive, accurate, precise and specific for the simultaneous estimation of all the three drugs in bulk and pharmaceutical dosage forms and for routine analysis in stability studies of these drugs.
Conclusion
The HPLC method developed and validated allows a simple and fast quantitative determination of Hydrochlorothiazide, Amlodipine besylate and Telemisartan from its formulation. All the validation parameters were found to be within the limits according to the ICH guidelines. The proposed method was found to be specific for the drugs of interest irrespective of the excipients present and the method was found to be suitable for the routine analysis of the marketed formulation.
Acknowledgements
The authors are thankful to the Mylan Labs limited, Hyderabad for providing gift sample of Telmisartan, Amlodipine Besylate and Hydrochlorothiazide. The authors also extend their thanks to the management of R.V.R&J.C College of engineering and Chebrolu Hanumaiah Institute of Pharmaceutical Sciences, Chowdavaram, Guntur for providing congenial facilities and support to carry out this research work.
References
- The Merck Index 14th Edition, Merck research laboratories 4581, 491, 9129.
- Manish Maladkar , Vijay Kumar Verma.Open Journal of Internal Medicine, 2012, 2, 67-71.
- Indian Pharmacopeia. Amlodipine Besylate, Hydrochlorothiazide; Govt. of India, Ministry of Health and Family Welfare, Controller of Publication: New Delhi, 2007.
- K. Muthu, R. Sankhla, S. Gupta, A. A. Smith, and R. Manavalan, Journal of Chemical Research,, 2010 , 12, 43–52.
- Vijaya V, Vrushali T, Vrushali K, Dhole SN, Int. J. Chem. Res.2011, 2, 7 – 10.
- Mallikarjuna Rao N, GowriSankar D, Int. J. Pharm. Pharm Sci. Res, 2011 ,1, 1 – 5.
- Patel BA, Captain AD.Indo American Journal of Pharmaceutical Research, 2014,4(6), 3031-3038.
- G. Saravanan, M. Padmaja, A. Lakshmi Gowthami, Journal of Chemical and Pharmaceutical Sciences, 2014, 7(3), 224-228.
- ShivajiJadhav , MeghaRai, Kawde.P.B and Mazahar Farooqui ,Der Pharmacia Sinica, 2015, 6(4),13-18.
- RLC Sasidhar, S.Vidyadhara, B.Deepti, K.Tejaswi and J.Suhasini, Oriental Journal of Chemistry, 2014, 30 (4), 1815-1822.
- ICH Q2A; Guidelines on validation of analytical procedure; Definitions and terminology, Federal Register, 60, 11260 (1995).
- ICH Q2B; Guidelines on validation of analytical procedure; Methodology, Federal Register, 60, 27464 (1996).
- ICH harmonized tripartite guideline, stability testing of new drug substances and products, Q1A (R2), 1‑15 (2003).
- Kishore kumar.H, S.Phani Kumar Reddy. International Research Journal of Pharmacy, 2013, 4(5), 78-84.
- Reddy,G.S; Reddy,S.L.N.P; Reddy,L.S. Orient.j.chem.,2013,29(4),1371-1380.
- Reddy,L.S; Reddy,S.L.N.P; Reddy,G.S. Orient.j.chem.,2014,30(3),1243-1251

This work is licensed under a Creative Commons Attribution 4.0 International License.









