Synthesis, Characterisation and Structural Studies of Complexes Containing Different Schiff Bases with Mn (Lll) And Mn (Ii) Transition Metals
Gulrez Nizami
Department of Chemistry Mohammad Ali Jauhar University Rampur u.p 244901, India
DOI : http://dx.doi.org/10.13005/ojc/290440
Article Received on :
Article Accepted on :
Article Published : 14 Jan 2014
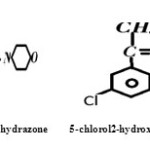
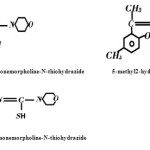
The Schiff bases 5-methyl2-hydroxyacetophenonemorpholine-N-thiohydrazide, 5-methyl2-hydroxyacetophenoneantipyrine 5-chloro2-hydroxyacetophenonemorpholine-N-thiohydrazone has reacted with MnII and MnIII to form co-ordination compounds having general formula [M (C14H19O2N3S) 3H2O] Cl; [M (C14H19O2N3S).3H2O]; [M (C20H20N3O2) 2] Cl; [M (C20H20N3O2) 2];[M(C13H14O2N3SCl).3H2O]Cl and [M(C13H14O2N3SCl].3H2 O] respectively. Where M=Mn III and Mn II. The adducts have been characterized on the basis of elemental analyses molar conductance, I.R , visible spectra, magnetic susceptibility measurement and TGA. The ligands behave in dibasic tridentate manner in 5-methyl2-hydroxyacetophenonemorpholine-N-thiohydrazone and 5-chloro2-hydroxyacetophenonemorpholine-N-thiohydrazone.While5-methyl2hydroxyacetophenoneantipyrine behaves in monobasic tridentate manner. All these compounds are paramagnetic in nature and have octahedral geometry.
KEYWORDS:coordination compounds; molar conductance and magnetic susceptibility.
Download this article as:| Copy the following to cite this article: Nizami G. Synthesis, Characterisation and Structural Studies of Complexes Containing Different Schiff Bases with Mn (Lll) And Mn (Ii) Transition Metals. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Nizami G. Synthesis, Characterisation and Structural Studies of Complexes Containing Different Schiff Bases with Mn (Lll) And Mn (Ii) Transition Metals. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1595 |
INTRODUCTION
Schiff bases have been used as chelating ligands in the field of co-ordination chemistry and their metal complexes are of great interest for many years It is well known that N and S atoms play a key role in the co-ordination of metals at the active sites of numerous metallobiomolecules(1). Schiff base metal complexes have been widely studied because they have industrial,antiungal,antibacterial,anticancer and herbicidal applications(2,3). They serve as models for biologically important species and find applications in biomimetic catalytic reaction. Chelating ligands containing N, O and S donor atoms show broad biological activity and are of special interest because of the variety of ways in which they are bonded to metal ions. It is known that the existence of metal of metal ions bonded to biologically active compounds may enhance their activities (4, 6).The variety of possible Schiff base metal complexes with wide choice of ligands and coordination environments, has prompted us to undertake research in this area (7). The survey of chemical literature has revealed that Schiff bases possess strong ability to form complexes (8). The Schiff bases have been investigated because of easy synthesis & remarkable versatility towards coordinating ability with transition metals (9, 10). The Schiff base compounds have received much attention owing to their significant biological activities and medicinal properties such as anti oxidative (11), anti tumor (12), antiviral (13), antineoplastic (14) and antimicrobial activities (15).
The complexes of different Schiff bases (5- methyl 2- hydroxy acetophenone morpholine-N-thiohydrozone,5-chloro2hydroxyacetophenonemorpholine-N-thiohydrazone and 5-methyl 2-hydroxy acetophenone antipyrine) with Mn (III) and Mn (II) have been synthesized in the lab. The structures of these complexes have been proposed on the basis of elemental analyses, electronic and IR spectral data and magnetic properties
EXPERIMENTAL
The carbonyl compounds and the compounds of amino group used for the preparation of Schiff bases were of AR grade or equivalent purity. The carbonyl compounds (5-methyl2-hydroxy acetophenone and 5-chloro2-hydroxy acetophenone) and amino compounds (morpholine-N-thiohydrazide and 4-amino antipyrine) were used for the preparation of Schiff base. The metal salts used were MnCl2 and MnCl3 (Glaxo). Other chemicals like ethanol, methanol (Glaxo) DMF (Rankem), DMSO (Glaxo) etc were of highest purity and used as such.
 |
Click here to View figure |
Infrared spectra and elemental analyses were carried out at CDRI (Lucknow). The magnetic susceptibility was measured by GOUY method. CuSo4 .5H2O was used as calibrant. U.V-visible spectra were recorded with the help of Beckmans-DU at Chemistry Deptt. BareillyCollegeBareilly. The conductivity measurement was carried out at room temperature and 10-3M dilution using conductivity bridge model 910. The thermo gravimetric analysis was carried out at G.N.D.UniversityAmritsar. Anti bacterial and anti fungal activities were carried out at IVRI Bareilly.
PREPARATION OF LIGANDS:
The Schiff bases were prepared by the condensation of Carbonyl compounds and compounds containing amino group. 1.41 gm of Morpholine N-Thiohydrazide was dissolved in Ethanol and refluxed on water bath for half an hour. Then 1.50 gm 5- methyl 2- hydroxy acetophenone was added to it and refluxed for about five hours. . 1.41 gm of Morpholine N-Thiohydrazide was dissolved in Ethanol and refluxed on water bath for half an hour. Then one mole 5- chloro 2- hydroxy acetophenone was added to it and refluxed for about five hours. Similarly 2.06 gm of 4-Amino antipyrine was dissolved in methanol. Then 1.50 gm 5- methyl 2- hydroxy acetophenone was added to it and refluxed for about five hours. The crystal of ligand so obtained was purified by recrystalization. The purity of crystal was checked by TLC and melting point. The ligands were characterized by elemental analysis, electronic and I-R spectra.
 |
Click here to View figure |
PREPARATION OF COMPLEXES:
All the metal complexes were synthesized by adding respective metal salts solution drop wise to the solution of the ligand (5- methyl 2- hydroxy acetophenone morpholine –N- thiohydrozone, 5chloro 2 hydroxy acetophenone morpholine N thiohydrozone, 5 methyl 2 hydroxy acetophenone anti pyrine) prepared. The precipitate obtained was filtered and washed with suitable solvents (DMSO and DMF) and dried in vacumm desiccator over fused P4O10
RESULTS AND DISCUSSION
The analytical data of 5-methyl 2-hydroxy acetophenone morpholine N thiohydrozone Mn III chloride complex and 5- methyl 2- hydroxy acetophenone morpholine N thiohydrozone Mn II complexes indicated 1/1 metal-ligands stoichiometry for the complex and hence the molecular formulae comes out to be [Mn(C14H19O2N3S)3H20]+Cl– & [Mn(C14H19O2N3S)3H20] respectively. Similarly 5-chloro 2- hydroxy acetophenone morpholine N thiohydrozone Mn III chloride, 5chloro 2 hydroxy acetophenone morpholine N thiohydrozone Mn II complexes also indicated M/L ratio 1/1. Hence molecular formulae of complex comes out to be [Mn(C13H14N3O2SCl)3H2O]Cl and [Mn(C13H14N3O2SCl)3H2O] respectively. The analytical data indicated 1:2 metal-ligand ratio for the 5-methyl2-hydroxyacetophenoneantipyrineMn(III)chloride complex and 5-methyl2-hydroxyacetophenoneantipyrineMn(II) complex. Hence the M.F formula of the adduct may be written as [Mn(C20H20N3O2S)2]Cl & [Mn (C20H20N3O2S)2] respectively. The m.p of the metal complexes was found to be much higher than that of the ligand which indicated the formation of the adduct.
The values of molar conductance in DMF and DMSO AT 10-3 dilution and 250C indicated 1:1 electrolytic nature of 5-methyl 2- hydroxy acetophenone morpholine-N-thiohydrazoneMn(III)chloride and 5-chloro2-hydroxy acetophenone morpholine N-thiohydrozone Mn (III) chloride complex. While Mn (II) complexes of respective ligands were found to be non electrolytic in nature. 5-methyl 2-hydroxy acetophenone antipyrine Mn (III), chloride complex indicated 1:1 electrolytic nature and Mn (II) complex of respective ligand indicated non electrolytic nature of the complex. The values of magnetic moment Came out to be about 5.93 B.M for Mn+3 and About 4.70 for Mn+2 complexes.(23,24)
The electronic spectra of Mn (III) complexes recorded in DMF, exhibited four bands in the range of 13590 to 14084 cm-1 , 16450 to1695 cm-1 ,18220 to 20202 cm-1 and 25600 to 25641 cm-1 (16) may be assigned respectively to the transitions 5B1 →5A1 , 5B1 →5B1 , 5B1 →5E & LMCT.
The electronic spectra of Mn (II) complexes were also recorded in DMF, exhibited three bands at 17000, 24000 and28000 cm-1 corresponding to the transitions 6A1g →4T1g (ν1), 6A1g →4T1g (ν2) and 6A1g →4Eg (ν3) respectively (17). These are characteristics of octahedral geometry (18).
The IR spectra of complexes of 5-methyl 2-hydroxy acetophenone morpholine N thiohydrozone and 2-hydroxy 5-chloro acetophenone morpholine N thiohydrozone indicated di-basic tri-dentate nature of the ligand as revealed by the comparison of this spectrum with that of the ligand shows band due to phenolic OH and νc=s, which disappeared in the IR spectra of the complexes and a new band has appeared at the range of 745 to 760 cm-1. This suggested the coordination of metal ion with the ligand through thiol-sulphur and phenolic oxygen via deprotonation. In addition to that the lowering of νc=N in the IR spectrum complexes by 15 to 20 cm-1 indicated the coordination of azomethine N, which is further substantiated by the appearance of azine chromophore at 1610 to 1615cm-1 (19). This indicated that ligand is behaving in dibasic tridentate manner coordinating through oxygen, nitrogen and sulphur atoms.
The IR spectrum of the complexes exhibited some new bands. The band at 3100 to 3480 cm-1 may be attributed to νOH of coordinated water. This is further supported by the band at 810 to 880 cm-1 may be due to rocking & wagging modes of coordinated water molecules. (20, 21).
This inference of IR spectrum regarding the presence of coordinating water molecule is further supported by TGA. The thermogram shows the loss of three water molecules above 150 to 1700c. In the far IR region two bands were observed at the range of 400 to 480 & 500 to 590 cm-1 which may be assigned to νM-N & νM-O vibrations respectively (22).
The i.r. spectra of the complexes of 5- methyl2-hydroxyacetophenoneantipyrine on comparison with that of ligands indicated co-ordination through cyclic C=O, phenolic oxygen and azomethine nitrogen. This indicated monobasic tridentate nature of the ligand. The band due to νOH present at 3500 cm-1 which disappeared on complexation and appearance of new band at 1250 to 1270 cm-1 which indicated the bonding of ionized phenolic oxygen to the metal ion (25). The strong band at 1590 cm-1 characterstics of the azomethine group in the free Schiff base has shifted to lower frequency of 1560-1570 cm-1 in the complexes indicating that azomethine N is one of the co-coordinating atoms in schiff base. The band observed at 1656 cm-1 characterstics of the cyclic carbonyl group of the pyrazolone
ring of free Schiff base has shifted to lower frequency in the i.r range of 1610-1620 cm-1 in the complexes, indicated the co ordination of the above group with metal ion(26). This is further supported by the formation of new bands in i.r spectrum of the complex at 460-475 cm-1 and 410-415 cm-1. Which are due to υ M-O and υM-N bands respectively.(27)
CONCLUSION
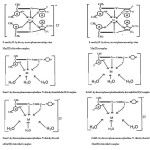
On the basis of above mentioned facts the complexes may be tentatively assigned the following octahedral geometry.
 |
Click here to View figure |
CHARACTERISATION OF COMPLEXES PREPARED
 |
Table 1: Click here to View table |
REFERENCES
- L.J Bellamy and R.F Branch, .chem soc. 4491(1954) .
- G.BDeacon and R.J Phillips co-ord. chem.. Rev33 . 227 (1980) .
- J.B Pilmoor, J.K Gaunt-Prog. Pestic Biochem 1:147 (1981) .
- J.Hollec, K. Handler, T.K Chatopadhyay, B.Majje and A.K. Kumar, coll. Czech chem. Commun 51 1100(1986) .
- S. Carpel , J.Am chem. Soc, 116 6037 (1994) .
- K.C Mooley, K.Quill and I.W Mowell, chem. Soc, Dalton Trance 101(1987) .
- Pires dos Santos. M.L.Alairo A.F.Mangrich A.S and FerreiraA.M.C, J.Inorg. Biochem, 71,(1998) .
- Yang Z.Y, Yang R.D Lif.S and YuK.B, Polyhedron, 19 , 2599 (2000) .
- Das A.TrousdaleM.D, Ren S. and Lien E.J , Antiviral Res, 44, 201 (1999) .
- Sur B, Chatterjee S.P, SurP, MaityT and Roy choudhry S, Oncology, 47, 433 (1990) .
- Fiora vantiR, Biava,M , .DonnarramaS., SimonettiG.C, VillaA., PugliaA.P ,Deiddo D. MaulloC and PomeiR.FarmacoI.L , 51, 643 (1996) .
- S.Goyal and K.Lal J. Indian chem.. soc. 66. 477 (1989) .
- B.Dash ,P.KMahapatra, D.Panda and J.M.Palnaik, J.Ind. chem.. soc. 61. 1061 (1984) .
- R.K. Parashar , R.C.Sharma, A.Kumar and G.Mohan, Inorg. Chem.. Acta. 151 201 (1988) .
- M.B.Davies , Coord chem.. Rev., 1997, 16427, D.R. Smith, Coord chem.., Rev. 1998, 172, 457, S.Yamada , Coord chem.., Rev., 1999, 190, 537 .
- R.Mukhopadhyay, S.Bhattacharjee. andR.Bhattacharya. Proc, Ind. Acad chem. Sci. ,1996 , 108, 193 .
- Zoeb A.Filmwala,SanjayM.Nandawadekar and R.M Patil, AAs.J.chem vol 19,No.6 (2007) 4697-470.
- Ferror J.R, Low Frequency vibrations of Inorganic & co-ordination compounds (Plenum, New York) ,!(1971) , pp-90 .
- Biradar N S & Kulkarni VH, inorg.chem, 33(1972) 2451.
- J.J Charette,Spectrochem, Acta 19: 1275 (1963).
- Nakamotto K, Infrared& Raman Spectraof inorg.co.ordination compounds5th Edn (widely-interscience NewYork) 1997.
- P.R.Mandlik,S.R.Aswale.J.Ind.Chem.Soc.,79689(2002).
- S.R.Aswale,P.R.Mandlik,S.S.Aswale and A.S.Aswar,Ind.J.of Chem.,42 322-326(2003).
- C.J.Carrano and J.A Bonandies,J.Am.chem.soc.108,4088 (1986).
- J.Chakravarty,S.Dutta,A.Dey and A.Chakravarty,J.Chem.Soc.Dalton Trans.,557(1994).
- S.Yadav,O.Pandey, P.Sen and S.K.Gupta, J.Coord.Chem.,38 107(((1996).
- Kriza .A, RessiA, ForeaS and Maghea A ,Polish J. chem.., 74(2000) 585 .J.J Charette

This work is licensed under a Creative Commons Attribution 4.0 International License.









