The Effects of TiO2 Nanoparticles over Time on the Physical and Mechanical Properties of White Cotton Fabrics and Fabrics Died with Reactive Dyes
Mohammadreza Mirkhan1, Mohammad Esmail Yazdanshenas2, Ramin Khajavi1 and Mohammad Mirjalili1
1Textile Engineering, Department of Polymer and Textile, Yazd Branch, Islamic Azad University, Yazd, Iran
2Department of Polymer and Textile, South Tehran Branch, Islamic Azad University, Tehran, Iran
Corresponding author email: enoor_2006@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320245
Article Received on :
Article Accepted on :
Article Published : 26 Apr 2016
Today, nano particles of titanium are used mainly in the textile industry. Examples of it can be named as cultivation of nano particles of titanium on polyester cotton fabrics, with features as white-washing, self- cleaning and also the effect of TiO2 on the dyed textiles with natural dyes as well as the effect of commodities reactive to increase the brightness and transparency of them. But, as this procedure has the added benefits, it will sure have some disadvantages and thus, the aim of this project is to study the effects of nano- TiO2 in the passage of time, at different times over the physical and mechanical properties of checked cotton fabrics. And even the study of dyed fabrics with natural dyes in reactive so that it can examine the beneficial and harmful effects of the degradation and also check the results on the Cotton Nano TiO2 Fabric.
KEYWORDS:textile; dyed fabrics; Nano Tio2 Fabric; nanoparticles of titanium
Download this article as:| Copy the following to cite this article: Mirkhan M, Yazdanshenas M. E, Khajavi R, Mirjalili M. The Effects of TiO2 Nanoparticles over Time on the Physical and Mechanical Properties of White Cotton Fabrics and Fabrics Died with Reactive Dyes. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Mirkhan M, Yazdanshenas M. E, Khajavi R, Mirjalili M. The Effects of TiO2 Nanoparticles over Time on the Physical and Mechanical Properties of White Cotton Fabrics and Fabrics Died with Reactive Dyes. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15556 |
Introduction:
The main challenge for the textile industry today is modify production methods, so they are more ecologically friendly at a competitive price, by using safer dyes and chemicals and by reducing cost of effluent treatment/ disposal (Pokharna & Shrivastav, 2013).
Dyes are introduced into the environment as a result of several man-made activities (Ram et. al., 2012). The textile industry accounts for the largest consumption of dyestuffs, at nearly 80% (Easton J.R). The use of solar radiation for the photocatalytic oxidation of organic contaminants in waste water is fast developing application (Pokharna & Shrivastav, 2013).
In this regard, the heterogeneous photocatalytic oxidation processes has been extensively used in the literature for the degradation of dyes (Azbar et al.,2004 & Wu, et al. 2006). Photocatalytic treatments are based on in situ generation of highly reactive hydroxyl radicals. These radicals are high oxidant species; they attack the most of organic molecules. They are also characterized by low selectivity of attack which is useful characteristic for an oxidant used in wastewater treatment. In the last decade, most attention has been given to Tio2 due to its high photocatalytic activity, low cost, nontoxicity and high stability in aqueous solution [Hu et al. 1999). TiO2 is generally considered to be the best photocatalyst and has the ability to detoxificate water from a number of organic pollutants (Kansal et al. 2007). Many studies have been reported related with textile dye and textile wastewater degradation using Tio2 as a catalyst [Guendy, 2009, Soutsas, et al. 2010, Kuo & Hu, 2006, and Poulios & Aetopoulou, 2010). Only a handful studies have been attempted which compare the efficiency of different catalysts for a particular dye under identical conditions (Barakat, 2015).
In this project it is tried to assess the impact of the TiO2nanoparticles over the passage of time, at different times on the physical and mechanical properties of cotton fabrics. And even it tries to study dyed fabrics with natural dyes in reactive dyes, so that it can check the degradation and also beneficial and harmful effects on the TiO2 Nano cotton fabric.
Coupling of two Semiconductors
Coupling of two semiconductors with different energy levels causes more effective separation of the charge. Hole created in the layer of cadmium sulfide capacity remains, but the electrons immigrate to the conduction band of titanium dioxide that has lower levels and causes the separation of the charge and improvement the efficiency of optical catalyst (Fig. 1)
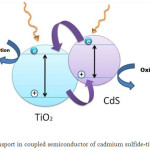 |
Figure 1: Charge transport in coupled semiconductor of cadmium sulfide-titanium dioxide Click here to View figure |
Research Goals
The overall objective of this study was to achieve the following results:
- Determining the decline of mechanical properties of fabric with nano- TiO2 particles over the time of at least 6 months
- Determining the effect of TiO2 nano particles on dyed fabrics with reactive dyes over time
- Comparing normal light radiation and UV light falling on the physical and mechanical properties of white and dyed fabrics as well as fabrics containing TiO2 nano particles
Hypotheses
It is assumed that nano- TiO2 particles for their photo-catalytic properties and because they result in oxidation and reduction reactions, they can affect the physical and mechanical properties of fibers, and that the fibers are classified in the organic materials category, this hypothesis is strengthened because organic materials are more sensitive in compare with oxidizers and regenerative inorganic materials.
2. On the other hand, since the TiO2 has photocatalytic properties, it can affect the stability of the light on colored fabrics. Especially, if light stability is tested by lamps with UV spectrum such as xenon lamps.
Data collection tools
Test collection tools in this research included: tables, sampling, laboratory and online databases and articles.
Findings from data analysis
Back from wrinkles
Figure 2 shows the crease recovery in the weft direction among the samples of white, yellow, white treated with TiO2and yellow treated with TiO2under UV and figure 3 shows the crease recovery in the warp direction among the samples of white, yellow, white treated with Tio2 and yellow treated with TiO2 under UV.
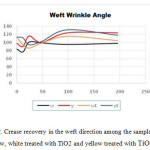 |
Figure 2: Crease recovery in the weft direction among the samples of white, yellow, white treated with TiO2 and yellow treated with TiO2 under UV Click here to View figure |
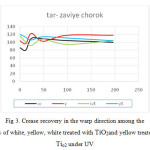 |
Figure 3: Crease recovery in the warp direction among the samples of white, yellow, white treated with TiO2and yellow treated with Tio2 under UV Click here to View figure |
Flexural stiffness
According to figure 4 and 5, flexural stiffness of white fabrics operations has decreased a lot with increasing duration of UV, but after 48 minute intervals, samples go on a uniformity process.
Samples with Tio2 have had more flexural stiffness.
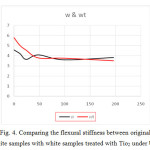 |
Figure 4: Comparing the flexural stiffness between original white samples with white samples treated with Tio2 under UV Click here to View figure |
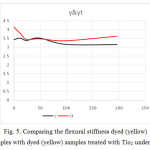 |
Figure 5: Comparing the flexural stiffness dyed (yellow) samples with dyed (yellow) samples treated with Tio2 under UV Click here to View figure |
Strength gauge
According to figures 6 to 11, the tensile strength and force and strain in dyed samples,
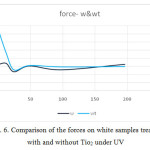 |
Figure 6: Comparison of the forces on white samples treated with and without Tio2 under UV Click here to View figure |
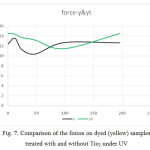 |
Figure 7: Comparison of the forces on dyed (yellow) samples treated with and without Tio2 under UV Click here to View figure |
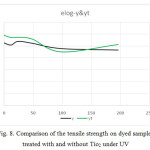 |
Figure 8: Comparison of the tensile strength on dyed samples treated with and without Tio2 under UV Click here to View figure |
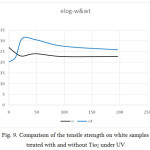 |
Figure 9: Comparison of the tensile strength on white samples treated with and without Tio2 under UV Click here to View figure |
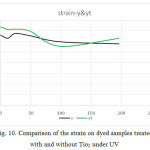 |
Figure 10: Comparison of the strain on dyed samples treated with and without Tio2 under UV Click here to View figure |
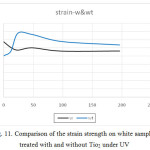 |
Figure 11: Comparison of the strain strength on white samples treated with and without Tio2 under UV Click here to View figure |
Conclusion
The results from the experiments are as follows:
- The results showed that Tio2 caused the white samples fading out more i. e. The presence of TiO2 in white samples has led to higher reflection.
- The presence of Tio2 has led to more reflection on the yellow stuff.
- UV operations in samples without Tio2 have increased the color strength.
- UV operations in the presence of Tio2 decreased the strength of color and led to reflectance in samples.
- The force level in dyed samples was higher and also, the samples with Tio2 had less force.
- The tensile strength is greater in dyed samples, but by adding Tio2 this trend has reversed. The strain in white samples was higher and by adding Tio2 this trend has been reversed.
- The tensile strength, force and strain in dyed samples, initially in a period of 48 minutes in samples with Tio2 were higher than in samples without Tio2. But after a period of 48 minutes, it was reversed. So that after 196 minutes, this trend continued almost to its original state with a slight slope. After 98 minutes, the trend goes on a stable process.
The samples containing Tio2 had more flexural stiffness.
References
- Azbar N., Yonar T. & Kestioglub K., Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dying effluent, Chemosphere, 2004., 55, 35-43.
CrossRef - Barakat M. A. Adsorption and photodegradation of Procion yellow H-EXL dye in textile wastewater over TiO2 suspension, Journal of Hydro-environment Research 2010.
- Easton J.R The dye maker’s view; Journal: color in dye house effluent; P. cooper Edu., Society of dyers and colorists: Bradford, England; 1995. 89; 9-12.
- Guendy H. R. Enhancing of Textile Wastewater Treatment Using Different Catalysts for Advanced Oxidation Process, Journal of Basic and Applied Sciences, 2009 4046-4052.
- Hu C. and Wang Y., “Decolorization and biodegradability of photocatalytic treated azo dyes and wool textile wastewater”, Chemosphere, 1999. 39: 2107-15,
CrossRef - Kansal S.K., Singh M. & Sud D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts, Journal of Hazardous Materials, 2007., 141, 3, 581-590.
CrossRef - Kuo W.S. and Ho P. H. solar photocatalytic decolorization of dyes in solution With TiO2 film, Dyes and Pigments, 2006, 71, 212-217.
CrossRef - Pokharna S. & Shrivastav R. Photocatalytic Treatment of Textile Industry Effluent Using Titanium Oxide. International Journal of Recent Research and Review, 2013.. 6, 2.
- Poulios I. & Aetopoulou I. Photocatalytic Degradation of the Textile Dye Reactive Orange 16 in the Presence of TiO2 Suspensions, Environmental Technology, 2010., 20, 479-487.
CrossRef - Soutsas K. , Karayannis V., Poulios I., Riga A., Ntampegliotis K., Spiliotis X., Papapolymerou G., Decolorization and degradation of reactive azo dyes via heterogeneous Photocatalytic processes, Desalination 2010 345-350.
CrossRef - Ram c. ,Ravi Kant Pareek R. K. & Singh V. Photocatalytic Degradation of Textile Dye by Using Titanium Dioxide Nanocatalyst, International Journal of Theoretical & Applied Sciences 2012. 4(2): 82-88.
- Wu C. , Chang C. & Kuo C. Decolorization of Procion Red MX-5B in electrocoagulation (EC),UV/TiO2 and ozone-related systems, Dye & pigment 2006 187-194.

This work is licensed under a Creative Commons Attribution 4.0 International License.









