A nano capacitor with graphene electrodes and Methane - (h-BN)insulator
Farrokh Roya Nikmaram
Department of Chemistry, Faculty of Science, Yadegar-e-Imam Khomeini (RAH) Shahre Rey Branch,Islamic Azad University, Tehran, Iran. Corresponding Author Email: nikmaram87@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320120
Article Received on :
Article Accepted on :
Article Published : 09 Mar 2016
Methan has a large potential to adsorb and diffuse among h-BN and graphene surfaces as the suitable dielectric. With this background the nanoscale dielectric capacitors have been widely studied due to their ability to store a high amount of energy. In this research, I have modeled one which is composed of two graphene layers including insulating medium of a h-BN layers which are filed out (Methane)n,m {n=m=7). It has been indicated thatthe Methane moleculeis the suitable gas for hetero-structures of the G/(h-BN)-(Methane)7,7/G capacitor compared to those nonpolar gases of 3 atoms . The quantum and coulomb blocked effects of h-BN/graphene including different numbers of Methane for multi dielectric properties of different thicknesses have been studied. We have shown that has appeared in a small thickness of capacitor due to number of layers and Methane atoms.
KEYWORDS:Capacitor; Graphene; Boron nitride sheet; Coulomb blockade Methane properties
Download this article as:| Copy the following to cite this article: Nikmaram F. R. A nano capacitor with graphene electrodes and Methane - (h-BN)insulator. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Nikmaram F. R. A nano capacitor with graphene electrodes and Methane - (h-BN)insulator. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14833 |
Introduction
The sp2-hybridized B-N and C-C bonding has very similar characteristics in mechanics, while great difference in optics and electronics. Essentially, the similar points result from the close positions of B, C and N in the Mendeleev Table, while the different ones result from the heterogeneous atoms. The bonding orbit p of B-N is mainly dominated by 2p orbit of N, while 2p orbit of B contributes mostly to the antibonding orbit p*.
The discovery of graphene in 2004[1], an atomically thin two-dimensional (2D) graphitic crystal, offers new opportunities to nano electronics and nano materials and has stimulated worldwide enthusiasm for the research of 2D nano materials [2].
Graphene has a relatively flexible molecular structure due to its electronic properties.It can be chemically fittedon top of a deposit metal atom [1-4], a deposit molecule can be fitted on its top [2], nitrogen and boron can be incorporated in its structure [3] and can have doped atom inside its sheet. This structure can be used in a wide area of researches such as electronics, capacitor, superconductors, batteries and diodes.The considerable characteristic of graphene is that it is a Dirac solid, with the electron energy being linearly dependent on the wave vector near the vertices of the hexagonal Brillouin zone [4]. A complete history for the rising of graphene has been given by Geim and Novoselov [5].
Graphene has studied in two-dimensional nanostructures, including carbon and hydrogen atoms with sp2-hybrid lattice structures. Graphene has a structure analogous to benzene and polycyclic aromatic hydrocarbons and sp2 hybrid, the chemistries of the compounds can be thought to be similar, graphene has many novel properties, such as high surface area, excellent electrical conductivity and electron mobility at room temperature, and has unique thermal and mechanical properties recent investigations have demonstrated that graphene may find tremendous applications in many areas [3,4].
The nanoscale capacitors have recently developed and achieved properties which are premierto other systems of energy storage. The nanoscale dielectric capacitors (NDC) consist of two metallic graphene layers separated by insulating h-BN thin layers which have been successfully used to simulate structures of graphene/h-BN/graphene [1-4].
Both theoretical and experimental studies on metallic graphene were focusedon understanding the dielectric properties of these structures and forming thin layers that can besteadas charge holding plates [2,4].Furthermore it was shown that graphene can preserve current densities six order of magnitude larger than copper [5].It has been theoretically [6]and experimentally [7] shown that h-BN layers of any thickness can be grown ongraphene layers and it is also possible to flourish perpendicular carbon on top of the sheets [8] and is important material for adsorption[9-14].
In this study, we consider a NDC model of G/(h-BN)m-(Methane)7,7/G capacitor composed with (h-BN)sheets Methane gas as an insulator using ab initio calculations within the density functional theory (DFT) and extended-huckel. Ournanoscale capacitor model is composed of a few hexagonal h-BN layers, which are stacked between two metallic graphene sheets as can be seen in scheme1.
The h-BN belongs to the same family of stacked hexagonal materials as graphene, which consists of atomically thin sheets held together by weak van der Waals forces. Its intra-layer network consists of strong covalent bonds, whose partial ionic character turn the system into an insulator.
Multilayers of h-BN are wide bandgap insulators which can vail as a dielectricmaterial betweenmetal-doped graphene layers.In addition, the sheets that are latticematched to graphene allow one to attain sturdy and high accuracy nanoscale spacing between two parallel metallic graphene plates, which can beset to favorable values.
Monajjemi and coworkers have studied of nanotube carbon and graphene as electronic devices such as LiBTs, capacitors, diode, various transistor and biological sensors both in solvent and vacuum media, widely. [15-131]
Since the thickness of separation of the capacitors can be less than 15 angstrom, the stored energy has to be calculated from the first principles. However, the available first-principle methods allow us to treat the distribution of onlyone kind of excess positive or negative charge at a time in the same situation.Therefore, the main problem in this kind of calculations is related to separating positive and negative charges on the plates of electrodes in acapacitor. In our model, the charge carriers are electrons themselves; while they exist in excess in one plate, they are unloaded from the other one.
Theoretical model
In this model, a small capacitor is made by creating an insulating layer of (h-BN)between two metal-doped graphene surfaces. We assume that the capacitor electrodes carry charges from one electrode towards the opposite side. So the initial energy stored in the electrostatic field between the capacitor plates is given by Ej=Q2/2c.
One of the most fundamental effects in nanoelectronics is related to the significant change in the energy when the electrons are transferred into a nanoscopic material region such as quantum dot, which results in what is known as “Coulomb Blockade”.
By letting the electrons tunnel through the insulating layer from the negative terminal to the positive terminal, such that the charge (Q+∆qe) resides on the top plate and (−Q−∆qe) resides on the bottom plate, the stored energy in this situation is now Ef= Q+∆q2/2c.
Althoughthe charge itself is quantized, the charge on the capacitor plates is polarized and not quantized.
The energy cannot be stored in the capacitor plates until a single electron tunnels through the insulator h-BN layers from the negative terminal to the positive terminal where the change in stored energy can be indicated as ∆Es= Ef-Ej= (∆q(Q+∆q/2/c)(1).
large voltage is in the range of ∆q/2c<∆V<+∆q/2c,that is,the tunneling current will only flow when a sufficiently large voltage |∆V|>|qe/2c| exists.
The tunneling resistance can be assumed to be RTun= ∆V/1(2).which is not ausual resistance, however,theoretically allowing electrons to cross the insulating junction as discrete occurrences where “I” is the resulting current due to the tunneling effect.Tunneling resistance is not an usual resistance, but an imaginary one, allowing the electrons to cross the insulating junction during “t= RtunCQ” where is the quantum capacitance and is a characteristic time associated with tunneling events which is considered to be the approximate lifetime of the energy state of the electrons (on one side of the barrier).
The Rtun should be finite and not too big, so that tunneling can practically take place. In this case, the charge is said to be well quantized and the capacitor is considered to be a tunnel junction.
In this work we have calculated the Rtun various capacitors {(including graphene electrodes composed with (h-BN)sheets and Methane gas as the insulators}as a function of the thickness of (h-BN)n and difference in potential energy barrier between the capacitor electrodes (Via the uncertainty relationship between time and energy, ΔE Δt ≥h/2 →Rtun ≥h/2Πq2e(4)
When the quantum well descends belowthe Fermi level, the electrons start to be accommodated in this quantum well and the excesselectrons in the graphene layers become sensitive to charge spilling into the vacuumspace of capacitor.
Ajayan and his coworkers havesuggested [7] that the observed abnormal increase in capacitance with decreasing size is due to the negative quantum capacitance for nano-systems. This effect arises from many-body interactions since the chemical potential of the electrons decreases with the increase of electron density. A few studies have reported different aspects of quantum capacitance; of particular importance are the study of Maccuci whom has reported a capacitance of radial quantum dots, Wang et al., on capacitance of atomic junctions [132] and another study on non-linear quantum capacitance [133].
The QM component is a development of the density of states of the metal electrodes, and their Thomas-Fermi screening lengths. Hence the hybrid capacitance of any nano-capacitor architecture is given as: 1/Cnet = 1/CQ1+ 1/CQ2+ 1/Cg (5) Where Cnet is the netcapacitance of the nano-capacitor, Cg is the classical (geometric) capacitance,CQ1 and CQ2 are the quantum capacitances, due to the density of states of the (Li, Be, B),doped graphene electrodes M1G, and M2G, respectively.
Computational Details
In this study, we have mainly focused on getting the results from DFT methods such as m06, m06-L, and extended-Huckelfor the non-bonded interaction of G/(h-BN)-(Methane)n/G which are monotonous through the comparison between different situations. The m062x, m06-L, and m06-HF are new methane hybrid DFT functional with a good correspondence in non-bonded calculations and are useful for calculating the energies of the distance between two fragments in a capacitor. The double ζ-basis set with polarization orbitals were used for doped graphene atoms while single ζ-basis sets with polarization orbitals were employed for the h-BN layers.Calculations were performed usingGaussian 09and GAMESS-US packages.
For a non-covalent interaction, B3LYP is unable to describe van der Waals interactions [134, 135] in capacitor systems by medium-range interactions, such as the interactions of two electrodes and dielectric sheets. The lack of ability of B3LYP and most other popular functional to correctly describe medium-range of exchange and correlation energy limits their applicability for distant non-bonded systems of two electrodes and dielectric thickness in a capacitor. Moreover, some recent studies have shown that inaccuracy for the medium-range exchange energies lead to large systematic errors in the prediction of molecular properties [136].
A monolayer of graphene was optimized and allowed to relax to its minimum energy structure.It contains 45 atoms.After relaxation, all C-C-C angles and C-C bond were calculated to be around 120º and 1.421Å, respectively, which are in good agreement with reported values [137].
Geometry optimizations and electronic structure calculations have been carried out using the DFT approach which is based on an iterative solution of the Kohn-Sham equation [138] of the density functional.
The Perdew-Burke-Ernzerh of [139] exchange-correlation (XC) functional of the generalized gradient approximation (GGA) is adopted. The dimension of capacitor has been set via three dimensionsas 5.655× 12.306×15.037 Å and the sheets are separated by various distances along the perpendicular direction to avoid interlayer interactions (scheme1). During all of the calculation processes, the partial occupancies were considered byusing the Blochcorrections [140].
The charge calculation methods based on molecular electrostatic potential (MESP) fitting are not well-suited for treating larger systems where some of the innermost atoms are located far away from the points at which the MESP is computed. In such a condition, variations of the innermost atomic charges will not lead to significant changes of the MESP outside of the molecule, meaning that the accurate values for the innermost atomic charges are not well-determined by MESP outside of the molecule.The representative atomic charges for molecules should be computed as average values over several molecular conformations. Although infinite graphene sheets are intrinsically metallic, our BG system exhibits an increase in the metallic properties. The interaction energy for capacitor was calculated in all items as indicated in the equation 9:ΔEs(eV)= {EG/(h-BN)m-(He)n/G-(2EG+E(h-BN)m+E(He)n)}+EBSSE (6) where “the ΔEs” is the stability energy of capacitor.
The electron density (Both of Gradient norm & Laplacian), value of orbital wave-function, electron spin density, electrostatic potential from nuclear atomic charges, electron localization function (ELF), localized orbital locator (LOL defined by Becke & Tsirelson), total electrostatic potential (ESP), as well as the exchange-correlation density, correlation hole and correlation factor, and the average local ionization energy.
Results and Discussion
Horizontal boron nitride (h-BN) in nature is an ideal electrical insulator that can be polarized by applying an external electric field; the number of h-BN layers between the graphene plates has been calculated and optimized as a suitable simulation of dielectrics.Furthermore, graphene and h-BN are two well-known single layer honeycomb structures, so the proposed model can be easily fabricated.
In this study,BN was chosen as a dielectric since it is an excellent spacer with a lattice constant close to that of graphene.We specifically studied the dielectric properties of G/(h-BN)(Methane)/G,including the number of h-BN respectively.
Similar to graphene, the anisotropic binding of h-BN allows for the formation of various layered structures. Long-range interlayer interactions play adominantrole incharacterizing the structural and mechanical properties of these systems and hence their performance in the simulation of our capacitor models.
The sp2-hybridized B-N and C-C bonding has very similar characteristics in mechanics, while great difference in optics and electronics. Essentially, the similar points result from the close positions of B, C and N in the Mendeleev Table, while the different ones result from the heterogeneous atoms. The bonding orbit p of B-N is mainly dominated by 2p orbit of N, while 2p orbit of B contributes mostly to the antibonding orbit p*. This implies a significant charge transfer from B to N, around 0.4 electrons. In contrary, there is no significant electron transfer in C-C bonding so the B-N bonding is partially ionic; while C-C bonding is covalent (This also leads to the different electron band structure).
The values of the distances between graphene layers capping h-BN layers, dielectric constants of the layered h-BN sheets (k), magnitude of the charges on the graphene plates,electrostatic properties using the SCF density, fitting point charges to electrostatic potential charges from ESP fit,the stability energy of capacitor (eV),various capacitances including the net capacitance and the potential difference between two electrodes of graphene plates are listed in tables {1-2}.
 |
Table 1: Charges, HOMO, LUMO and Gap energy of the capacitor including 7+7=14 molecules of Methane |
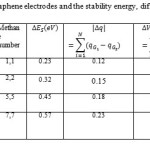 |
Table 2: The Charges of two graphene electrodes and the stability energy, difference potential, dielectric Click here to View table |
Gap energy and kinetic energy, potential energy, Laplacian of electron density and density of all electrons are depended to various number of He in the table1 which it can be exhibited the proper situations for He and number of h-BN plates in figs 1-5.
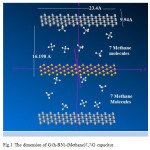 |
Figure 1: The dimension of G/(h-BN)-(Methane)7,7/G capacitor. Click here to View figure |
 |
Figure 2: The Capacitour versues 3 axis (Bohr) Click here to View figure |
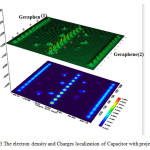 |
Figure 3: The electron density and Charges localization of Capacitor with projection Click here to View figure |
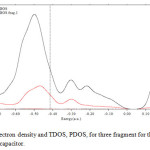 |
Figure 4: The electron density and TDOS, PDOS, for three fragment for theG/(h-BN)( Methane)/Gcapacitor. Click here to View figure |
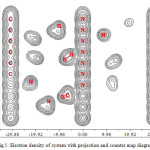 |
Figure 5: Electron density of system with projection and counter map diagram |
In addition, according to the electronic structures, we have considered two isolated graphene layers which are doped by these atoms in various distances. The atomic structures, interlayer spacing, relative positions of the layers and the cell parameters have been optimized.
Here we have considered the interlayer attraction using extended-Huckelforce fieldfor h-BN to describe its interlayer interactions including h-BN inter-layer potential, attractive componentsand the classical mono-polar electrostatic term that takes into account the partially ionic character of h-BN.
For long distances of dielectric thickness, the classical capacitance rule of the “ ” is adaptable. This adaptability does not go for short distances, which is attributed to the quantum size effect.We identified the dielectric permittivity as afunction of dielectric size through abinito calculations, CQ(F)x1020 = Δq(Q+Δq/2)/ΔES, Cnet (F)x1020 = CgCQ/CQ+2Cg and Quantum effect by Rtun (KΩ)>>hΔ2ESP, using themolecular dynamic simulation.
References
- Novoselov, KS.; Geim, AK.; Morozov, SV.; Jiang, D.; Zhang, Y.; Dubonos.SV.; Grigorieva, IV.; Firsob, AA, Science, 2004, 306,666-669
- Zhengzong, S.; Dustin, K J.; Tour, JM,J Phys Chem Lett, 2011, 2:2425-2432
- Nomura,K.; Ohta,H.; Ueda,K.; Kamiya,T.; Hirano,M.; Hosono,H,Science,2003 300 1269.
- Huangz, M. H.; Mao, S.; Feick, H.; Yan, H.;Wu, Y.; Kind, H.; Webber, E.; Russo, R.; Yang, P, Science, 2001, (292):1897-1899
- Geim,A.K.Science, 2009,324 , 1530 – 1534.
- Özçelik,V.O.; Cahangirov,S.; Ciraci,S, Phys. Rev. B,2012, 85 ,235456.
- Liu,Z.; Song,L.; Zhao,S.; Huang,J.; Ma,L.; Zhang,J.; Lou,J.; Ajayan, P.M., Nano Lett. 11 2011, 2032–2037.
- Ataca,C.; Ciraci,S, Phys. Rev. B, 2011, 83, 235417.
- Sadegh,H.; Shahryari-ghoshekandi,R.; Agrawal, S.; Tyagi,I.; Asif,M, Gupta,V.K,Journal of Molecular Liquids, 2015, 206, 151-158 .
- Gupta,V.K.; Tyagi,I.; Agrawal,S.; Sadegh,H.; Shahryari-ghoshekandi, R.; Yari,M.; Yousefi-nejat,O.;Journal of Molecular Liquids,2015, 206, 126-136
- Sadegh,H.; Shahryari-ghoshekandi,R.; Tyagi,I.; Agrawal,S.; Gupta,V.K,Journal of Molecular Liquids,2015, 207, 21-27.
- Sadegh,H.: Zare,K.; Maazinejad,B.; Shahryari-ghoshekandi,R.; Tyagi,I.; Agrawal,S.; Gupta,V.K,Journal of Molecular Liquids, 2016,215, 221-228.
- Zare,K.; Sadegh,H.; Shahryari-ghoshekandi,R.; Asif,M.; Tyagi,I.; Shilpi Agrawal, Gupta,V.KJournal of Molecular Liquids, 2016, 213, 345-350 (2016)
- Omid Moradi, O.; Gupta,V.K.; Agrawal, S.; Tyagi,I.; Asif,M.; Hamdy Makhlouf, A.S.; Sadegh,H.; Shahryari-ghoshekandi,R.;Journal of Industrial and Engineering Chemistry, 2015, 28, 294-301
- Monajjemi, M.; Lee, V.S.; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F. J. Phys.Chem C. 2010, 114, 15315
- Monajjemi, M.Struct Chem.2012, 23,551–580
- Monajjemi, M.; Chegini, H.; Mollaamin, F.; Farahani, P. Fullerenes, Nanotubes, and Carbon Nanostructures. 2011,19, 469–482
- Monajjemi, M .; Afsharnezhad ,S.; Jaafari , M.R.; Abdolahi ,T.; Nikosade ,A.; Monajemi ,H.; Russian Journal of physical chemistry A, 2007,2,1956-1963
- Monajjemi, M.;Baei, M.T.;Mollaamin, F. Russian Journal of Inorganic Chemistry. 2008, 53 (9),1430-1437
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
- Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013,117,1670 −1684
- Mollaamin, F.; Monajjemi, M, Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
- Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22(4), 673-692
- Mollaamin, F.; Varmaghani, Z.; Monajjemi, M, Physics and Chemistry of Liquids. 2011, 49 318
- Nafisi, S.;Monajemi, M.;Ebrahimi, S. Journal of Molecular Structure. 2004,705 (3)35-39
- Fazaeli, R.; Monajjemi, M.; Ataherian, F.; Zare, K. Journal of Molecular Structure: THEOCHEM.2002, 581 (1), 51-58
- Monajjemi, M.; Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.; Russian Journal of Physical Chemistry A , 2008 , 82 (13), 2277-2285
- Monajjemi, M.; Seyed Hosseini, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21, 381–393
- Monajjemi, M.; Faham, R.; Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures,2012 20, 163–169
- Mollaamin, F.; Najafi, F.; Khaleghian, M.; Khalili Hadad, B.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 201119, 653–667
- Mollaamin, F.; Baei, MT.; Monajjemi, M.; Zhiani, R.; Honarparvar, B.; Russian Journal of Physical Chemistry A, Focus on Chemistry, 2008, 82 (13), 2354-2361
- Monajjemi, M. Chemical Physics. 2013, 425, 29-45
- Monajjemi, M.; Heshmat, M.; Aghaei, H.; Ahmadi, R.; Zare, K. Bulletin of the Chemical Society of Ethiopia, 2007,21 (1)
- Monajjemi, M.;Honarparvar, B. H. ; Haeri, H. ; Heshmat ,M.; Russian Journal of Physical Chemistry C. 2006, 80(1):S40-S44
- Monajjemi, M.; Ketabi, S.; Amiri, A. Russian Journal of Physical Chemistry, 2006, 80 (1), S55-S62
- Yahyaei, H.; Monajjemi, M.; Aghaie, H.; K. Zare, K. Journal of Computational and Theoretical Nanoscience. 2013, 10, 10, 2332–2341
- Mollaamin, F.; Gharibe, S.; Monajjemi, M. Int. J. Phy. Sci, 2011, 6, 1496-1500
- Monajjemi, M.; Ghiasi, R.; Seyed Sadjadi, M.A.Applied Organometallic Chemistry,2003,17, 8, 635–640
- Monajjemi, M.; Wayne Jr, Robert. Boggs, J.E. Chemical Physics.2014, 433, 1-11
- Monajjemi, M.; Sobhanmanesh, A.; Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures, 2013, 21 47–63
- Monajjemi, M.; Mollaamin, F. Journal of Computational and Theoretical Nanoscience, 2012, 9 (12) 2208-2214
- Monajjemi, M.; Honarparvar, B.; Nasseri, S. M. .; Khaleghian M. Journal of Structural Chemistry. 2009, 50, 1, 67-77
- Monajjemi, M.; Aghaie, H.; Naderi, F. Biochemistry (Moscow).2007,72 (6), 652-657
- Ardalan, T.; Ardalan, P.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 687–708
- Mollaamin, F.; Monajjemi, M.; Mehrzad, J. Fullerenes, Nanotubes, and Carbon Nanostructures. 2014, 22: 738–751
- Monajjemi, M.; Najafpour, J.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21(3), 213–232
- Monajjemi, M.; Karachi, N.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 643–662
- Yahyaei, H.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures.2014, 22(4), 346–361
- Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics, 2013, 19, 155–164
- Monajjemi, M.; Mollaamin, F. Journal of Cluster Science, 2012, 23(2), 259-272
- Tahan, A.; Monajjemi, M. Acta Biotheor,2011, 59, 291–312
- Lee, V.S.; Nimmanpipug, P.; Mollaamin, F.; Kungwan, N.; Thanasanvorakun, S..; Monajjemi, M. Russian Journal of Physical Chemistry A, 2009, 83, 13, 2288–2296
- Monajjemi, M.; Heshmat, M.; Haeri, HH, Biochemistry (Moscow), 2006, 71 (1), S113-S122
- Monajjemi, M.; Yamola, H.; Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 595–603
- Mollaamin, F.; Layali, I.; Ilkhani A. R.; Monajjemi, M. African Journal of Microbiology Research .2010, 4(24) 2795-2803
- Mollaamin, F.; Shahani poor, p K. .; Nejadsattari, T. ; Monajjemi, M. African Journal of Microbiology Research. 2010, 4(20) 2098-2108
- Monajjemi, M.; Ahmadianarog, M. Journal of Computational and Theoretical Nanoscience. 2014, 11(6), 1465-1471
- Monajjemi, M.; Jafari Azan, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures.2013, 21(6), 503–515
- Mollaamin, F.; Monajjemi, M.Physics and Chemistry of Liquids .2012, 50, 5, 2012, 596–604
- Monajjemi, M.; Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
- Khaleghian, M.; Zahmatkesh, M.; Mollaamin, F.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2011, 19(4): 251–261
- Monajjemi, M.; Baheri, H.; Mollaamin, F. Journal of Structural Chemistry.2011 52(1), 54-59
- Mahdavian, L.; Monajjemi, M.; Mangkorntong, N. Fullerenes, Nanotubes and Carbon Nanostructures, 2009, 17 (5), 484-495
- Monajjemi, M., Mahdavian, L., Mollaamin, F. Bull. Chem. Soc. Ethiop. 2008, 22(2), 277-286
- Monajjemi, M.; Afsharnezhad, S, Jaafari, M.R..; Mirdamadi, S..; Mollaamin, F..; Monajemi, H. Chemistry .2008, 17 (1), 55-69
- Monajjemi, M.; Mollaamin,F.; Gholami, M. R.; Yoozbashizadeh,H.; Sadrnezhaad, S.K.; Passdar, H.;Main Group Metal Chemistry,2003, 26, 6, 349-361
- Monajjemi, M.; Azad ,MT.; Haeri, HH.; Zare, K.; Hamedani, Sh.; JOURNAL OF CHEMICAL RESEARCH-S.2003, (8): 454-456
- Monajjemi, M.; Najafpour, J. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22(6): 575–594
- Monajjemi, M.; Noei, M.; Mollaamin, F.Nucleosides, Nucleotides and Nucleic Acids. 2010 29(9):676–683
- Ghiasi, R.; Monajjemi, M.Journal of Sulfur Chemistry .2007, 28, 5, 505-511
- Monajjemi, M.; Ghiasi, R.; Abedi, A. Russian Journal of Inorganic Chemistry.2005, 50(3), 382-388
- Monajjemi, M. .; Naderi, F.; Mollaamin, F.; Khaleghian, M. J. Mex. Chem. Soc. 2012, 56(2), 207-211
- Monajjemi, M.; Farahani, N.; Mollaamin, F. Physics and Chemistry of Liquids, 2012, 50(2) 161–1774.
- Monajjemi, M.M. Journal of Computational and Theoretical Nanoscience .2013, 10(10), 2473-2477
- Monajjemi , M.; Honaparvar , B.; Khalili Hadad ,B.; Ilkhani ,AR.; Mollaamin, F. African Journal of Pharmacy and Pharmacology .2010, 4(8), 521 -529
- Monajjemi, M. Theor Chem Acc, 2015, 134:77 DOI 10.1007/s00214-015-1668-9
- Monajjemi, M. Journal of Molecular Modeling, 2014, 20, 2507
- Monajjemi , M.; Honarparvar, B.; Monajemi, H.;. Journal of the Mexican Chemical Society, 2006, 50 (4), 143-148
- Monajjemi, M.; Khaleghian, M.; Mollaamin, F. Molecular Simulation. 2010, 36, 11, 865–
- Ilkhani, Ali R.; Monajjemi, M. Computational and Theoretical Chemistry.2015 1074, 19–25
- Monajjemi, M. Biophysical Chemistry. 2015 207,114 –127
- Monajjemi, M., Moniri, E., Panahi, H.A , Journal of Chemical and Engineering Data.2001,1249-1254.
- Mollaamin, F.;Najafpour, J.;Ghadami, S.; Ilkhani, A. R.;Akrami, M. S.;Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 11 (5), 1290-1298
- Monajjemi, M.; Ghiasi, R.; Ketabi, S.; Passdar, H.; Mollaamin, F.Journal of Chemical Research . 2004, 1, 11.
- Monajjemi, M.; Heshmat, M.; Haeri, H.H. Biochemistry (Moscow).2006, 71, 113-122
- Monajjemi, M.; Heshmat, M.; Aghaei, H.;Ahmadi, R.; Zare, K. Bulletin of the Chemical Society of Ethiopia. 2007, 21, 111–116
- Monajjemi, M., Kharghanian, L., Khaleghian, M., Chegini, H.Fullerenes Nanotubes and Carbon Nanostructures.2014, 22, 8, 0.1080/1536383X.2012.717563
- Sarasia, E.M.; Afsharnezhad, S.; Honarparvar, B.; Mollaamin, F.; Monajjemi, M. Physics and Chemistry of Liquids. 2011, 49 (5), 561-571
- Amiri, A.; Babaeie, F.; Monajjemi, M.Physics and Chemistry of Liquids. 2008, 46, 4, 379-389
- Monajjemi, M.; Heshmat, M.; Haeri, H.H.; Kaveh, F. Russian Journal of Physical Chemistry A,2006, 80, 7, 1061-1068
- Monajjemi, M.; Moniri, E.; Azizi, Z.; Ahmad Panahi, H. Russian Journal of Inorganic Chemistry. 2005, 50, 1, 40-44
- Jalilian,H.; Monajjemi, M. Japanese Journal of Applied Physics. 2015, 54, 8, 08510
- Mollaamin, F.; Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2015, 12, 6, 1030-1039
- Felegari, Z.; Monajjemi, M. Journal of Theoretical and Computational Chemistry. 2015,14, 3, 1550021
- Shojaee, S., Monajjemi, M.Journal of Computational and Theoretical Nanoscience. 2015, 12, 3, 449-458
- Esmkhani, R.;Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2015. 12, 4, 652-659
- Monajjemi, M., Seyedhosseini, M., Mousavi, M., Jamali, Z.Journal of Computational and Theoretical Nanoscience. 2015 , 23 (3), 239-244
- Ghiasi, R.; Monajjemi, M.; Mokarram, E.E.; Makkipour, P. Journal of Structural Chemistry. 2008, 4 , 4, 600-605
- Mahdavian, L.; Monajjemi, M. Microelectronics Journal. 2010,41(2-3), 142-149
- Monajjemi, M.; Baie, M.T.; Mollaamin, F. Russian Chemical Bulletin.2010, 59, 5, 886-889
- Bakhshi, K.; Mollaamin, F.; Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2011, 8, 4,763-768
- Darouie, M.; Afshar, S.; Zare, K., Monajjemi, M. journal of Experimental Nanoscience.2013, 8, 4,451-461
- Amiri, A.; Monajjemi, M.; Zare, K.; Ketabi, S.Physics and Chemistry of Liquids. 2006, 44, 4, 449-456.
- Zonouzi, R.;Khajeh, K.; Monajjemi, M.; Ghaemi, N. Journal of Microbiology and Biotechnology. 2013, 23, 1 ,7-14
- Ali R. Ilkhani .; Majid Monajjemi, Computational and Theoretical Chemistry.2015, 1074 19–25
- Tahan, A.; Mollaamin, F.; Monajjemi, M. Russian Journal of Physical Chemistry A, 2009, 83 (4), 587-597
- Khalili Hadad, B.; Mollaamin, F.; Monajjemi, M, Russian Chemical Bulletin,2011, 60(2):233-236
- Mollaamin, F.; Monajjemi, M.; Salemi, S.; Baei, M.T. Fullerenes Nanotubes and Carbon Nanostructures, 2011, 19, 3, 182-196
- Mollaamin, F.; Shahani Pour.; K., Shahani Pour, K.; ilkhani, A.R.; Sheckari, Z., Monajjemi, M Russian Chemical Bulletin , 2012 , 61(12), 2193-2198
- Shoaei, S.M.; Aghaei, H.; Monajjemi, M.; Aghaei, M. Phosphorus, Sulfur and Silicon and the Related Elements. 2014, 189, 5; 652-660
- Mehrzad, J., Monajjemi, M., Hashemi, M , Biochemistry (Moscow).2014 , 79 (1), 31-36
- Moghaddam, N.A., Zadeh, M.S., Monajjemi, M. Journal of Computational and Theoretical Nanoscience , 2015 , Vol. 12, No. 3, doi:10.1166/jctn.2015.3736
- Joohari, S.; Monajjemi, M , Songklanakarin Journal of Science and Technology , 2015 , 37(3):327
- Rajaian, E., Monajjemi, M., Gholami, M.R, Journal of Chemical Research – Part S ,2002, 6, 1, 279-281
- Ghassemzadeh, L., Monajjemi, M., Zare, K, Journal of Chemical Research – Part S, 2003, 4, 195-199
- Mehdizadeh Barforushi,M.;Safari,S.;Monajjemi,M.J. Comput. Theor. Nanosci.2015, 12, 3058-3065.
- Mollaamin,F.; Ilkhani,A.; Sakhaei,N.; Bonsakhteh,B.; Faridchehr,A.; Tohidi,S.; Monajjemi,M.J. Comput. Theor. Nanosci.2015,12, 3148-3154
- Rahmati,H.; Monajjemi,M.J. Comput. Theor. Nanosci.2015,12, 3473-3481.
- Roghieh Tarlani Bashiz,R.; Monajjemi,M.J. Comput. Theor. Nanosci.2015, 12, 3808-3816
- Mehrabi Nejad,A.; Monajjemi,M.J. Comput. Theor. Nanosci. 2015, 12, 3902-3910.
- Monajjemi,M.; Bagheri,S.; Moosavi,M.S.; Moradiyeh,N.; Zakeri,M.;Attarikhasraghi,N.; Saghayimarouf,N.; Niyatzadeh,G.; Shekarkhand,M.; Khalilimofrad,M.S.; Ahmadin,H.; Ahadi,M.;Molecules 2015, 20, 21636–21657; doi:10.3390/molecules201219769
- Shabanzadeh,E.; Monajjemi,M.;J. Comput. Theor. Nanosci.2015, 12, 4076-4086
- Elsagh,A.; Jalilian, H.; Kianpour, E.; Ghazi, Mokri,H.S.;Rajabzadeh,M.; Moosavi,M.S.; Ghaemi Amiri,F.; Majid Monajjemi ,M.; J. Comput. Theor. Nanosci. 2015, 12, 4211-4218.
- Faridchehr, A.; Rustaiyan,A.; Monajjemi,M.;J. Comput. Theor. Nanosci.2015, 12, 4301-4314.
- Tohidi, S.; Monajjemi, M.; Rustaiyan, A.; J. Comput. Theor. Nanosci. 2015, 12, 4345-4351
- Ali Akbari Zadeh,M.; Lari,H.; Kharghanian,L.; Balali,E.; Khadivi,R.; Yahyaei,H.; Mollaamin,F.; Monajjemi, M.;J. Comput. Theor. Nanosci.2015, 12, 4358-4367.
- Dezfooli,S.; Lari,H.; Balali,E.; Khadivi,R.; Farzi,F.; Moradiyeh,N.; Monajjemi,M.;J. Comput. Theor. Nanosci.2015, 12, 4478-4488 .
- Jalilian,H.; Sayadian,M.; Elsagh,A.; Farzi,F.; Moradiyeh,N.; Samiei Soofi,N.; Khosravi,S.;. Mohammadian,N.T.; Monajjemi,M.J. Comput. Theor. Nanosci. 2015,12, 4785-4793.
- Farzi,F.; Bagheri,S.; Rajabzadeh,M.; Sayadian,M.; Jalilian,H.; Moradiyeh,N.; Monajjemi, M.;J. Comput. Theor. Nanosci.2015, 12, 4862-4872.
- Monajjemi,M.; Mohammadian, N.T,J. Comput. Theor. Nanosci. 2015, 12, 4895-4914
- Monajjemi, M., Chahkandi, B. Journal of Molecular Structure: THEOCHEM,2005,714 (1), 28, 43-60.
- Wang, B.; Zhao,X.; Wang,J. ; Guo,H.Appl. Phys. Lett, 1999, 74 ,2887-2889.
- Wang, J.; et al., Phys. Rev. Lett., 1998, 80 , 4277-4280.
- Zhao,Y.; Truhlar,D.G.;Theor Chem Account,2008, 120 , 215–241.
- Zhao,Y.; Truhlar,D.G.;Accounts of Chemical Research,2008, 41(2), 157-167.
- Check,C.E.; Gilbert,T.M.;J. Org. Chem,2005,70 , 9828–9834.
- Ao ,Z.; Yang,J.; S.Li.; JiangQ.;ChemPhys Lett,2008, 461(4), 276-279.
- Kohn,W. ; Sham,L. J. Phys. Rev. 1965,140 A , 1133-1138.
- Perdew,J.P. ; Burke ,K. Ernzerhof, Phys. Rev. Lett. 1996.77, 3865-3868.
- Blöchl, P. E. O. Jepsend , O. K. Andersen, Phys. Rev. B, 49 (1994) 16223.

This work is licensed under a Creative Commons Attribution 4.0 International License.









