Biosynthesis and antimicrobial activity studies of salicylalchitosan functionalized zinc oxide nanoparticles and comparative studies with its non-functionalized form
M. Jayandran1, M. Muhamed Haneefa2*and V. Balasubramanian2
1PG and Research Department of Chemistry, Government Arts College, Trichy-22, India
2Department of Chemistry, AMET University, Chennai-112, India
Corresponding Author Email: honey79101@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320180
Article Received on :
Article Accepted on :
Article Published : 07 Mar 2016
Biofunctionalization of nanomaterial is one such topic which is an advancement of current nanotechnology and to produce the non-toxic and more efficient antimicrobial agents. It becomes incorporated for development of biosynthetic and eco-friendly technology for nanomaterials synthesis. Therefore, the present study describes a synthesis of bioactive salicylalchitosan functionalized zinc oxide nanoparticles through a biosynthesis method and comparative studies of those nanoparticles with zinc oxide nanoparticles were undertaken.The antimicrobial activities of non-functionalized and bio functionalized zinc oxide nanoparticles were carried out against some well-known bacterial and fungal species. Synthesized materials were characterized by UV- Vis, FT-IR, SEM and TEM techniques and the results were reported.
KEYWORDS:Chitosan; Salicylalchitosan; Biosynthesis; Biofunctionalization; Antimicrobial activities
Download this article as:| Copy the following to cite this article: Jayandran. M, Haneefa M. M, Balasubramanian M. V. Biosynthesis and antimicrobial activity studies of salicylalchitosan functionalized zinc oxide nanoparticles and comparative studies with its non-functionalized form. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Jayandran. M, Haneefa M. M, Balasubramanian M. V. Biosynthesis and antimicrobial activity studies of salicylalchitosan functionalized zinc oxide nanoparticles and comparative studies with its non-functionalized form. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14753 |
Introduction
Biosynthesis of nanoparticles is a bottom up approach method where reduction/oxidation is the key reaction takes place. Plant extracts are rich sources of biomolecules, easily available,safe to handle and low cost which increases its part in the biosynthesis of nanoparticles. The microbial enzymes or the plant phytochemicals with anti-oxidant or reducing properties are usually the mainreason for reduction of metal compounds into their respective nanoparticles (1).
Biofunctionalization of nanoparticles can provide them with good biocompatibility for the immobilization of biomolecules and high specificity for biological recognition which led to produce a considerable effect in biological systems. Therefore, biofunctionalized nanomaterials are being given considerable attention in a multiple way of emerging fields of science and technology. However, pursuingappropriate biomaterials for the functionalization of nanomaterials is one of the crucial issues in this field. Various biomaterials were reported in the biosynthesis of nanoparticles (2-3). Chitosan is a naturally occurring biopolymer derived from chitin.Chitosan is a cost-effective and renewable material with many applications incosmetics, pharmaceuticals, food science and biotechnology (4-5).It has been attracted more attention for its unique properties, biodegradable,flexibility, non-hazardous, easy availability and used as a carrier in drug delivery. It has important biological properties such as biocompatibility, antifungal and antibacterial activity, wound healing ability, anti-cancer property, anti-cholesteremic properties and immune enhancing effect (6-7).
Many investigations have been focused on metal nanoparticles particularly gold, silver and platinum concerning antimicrobial applications to develop new and effective antimicrobial reagents. Considering the higher cost associated with the above materials, researchers are trying to find an antimicrobial agents by using the low cost materials and methods. By considering this factor alternative metal and metal oxide nanoparticles such as copper, nickel, magnesium, iron etc. were focused and reported in literature (8-9).
Zinc is an easily available and more reactive element and zinc nanoparticles are of great interest due to its essential constituent for cell growth and in inhibiting bacterial enzymes like dehydrogenase and certain protective enzymes such as thiolperoxidase and glutathione reductase. Various researches have shown that antimicrobial effect of Zn nanoparticles has been endorsed due to their good reactivity, which allows them to interact closely with microbial membranes. Zn nanoparticle acts efficiently to resist microorganisms and has a remarkable lengthy period of life than organic based disinfectants and antimicrobial agents (10-12).
Based upon the above discussion, this investigation was carried out to synthesize cost effective and possess antimicrobial active zinc oxide nanoparticles which was biofunctionalized with salicylalchitosanto enhance its antimicrobial activity. Firstly, salicylalchitosan was synthesized from the chitosan then zinc oxide nanoparticles (ZONP) were prepared by reducing zinc acetate with the help of lemon extract. Finally, salicylalchitosan was functionalized with synthesized zinc oxide nanoparticles. The antimicrobial results of biofunctionalized zinc oxide nanoparticles (ZNSC) showed efficient inhibition activity against bacterial strains and funguses tested.
Materials and Methods
The chemicals are of zinc acetate, aniline and ethanol were purchased from Merck (India) Ltd and all the chemicals and solvents used were of analytical reagent grade. Prawn shells of chitosan source was purchased from sea markets of Tuticorin, India. The biomaterialsalicylalchitosan was prepared by using salicylaldehyde and chitosan in the laboratory (13).
Collection of extracts
In this study fruits of citrus lemon were used as a bioreductant for the synthesis of zinc oxide nanoparticles. The main reason for selecting this, since lemons are a rich source of citric acid and ascorbic acid and it contains huge amounts of polyphenolic phytochemicalswhich is the excellent source for reducing agent (14-15). Fresh lemon fruits were cut into pieces and squeezed well to get about 10 ml pure extract. The extract was filtered by using whatman filter paper and the filtrate was collected and stored.
Synthesis of salicylalchitosan (SC)
According to Brine & Austin, 1981 (16); Muzzarelli & Jeuniaux, 1986 (17), the washed, dried and powdered prawn shells was de-mineralized with1M HCLand deprotienized with 1M sodium hydroxide at 60°C with constant stirring for an hour. The pink coloured solution was washed several times and dried then deacetylated with 40% sodium hydroxide for 2 hours at 60°C and the obtained dirty white precipitate of chitosan was washed several times and dried in oven at 600C for 3-4 hrs.
100 mg of dried chitosan powder was dissolved in 25 ml of acetic acid solution (0.5 M) and kept on magnetic stirrer for 2hrs.A mixture of 10 ml of salicylaldehyde (0.1 M) added to the chitosan mixture then kept on hot magnetic stirrer at 50-60°C for 6 hours and refluxed well. The obtained yellowish green precipitate of salicylalchitosan was washed well and dried under vacuum at 60°C for 12 hrs.
Synthesis of zinc oxide nanoparticles (ZONP)
The lemon extract (10 ml) was added drop wiseto the zinc salt (1 mmol) solution and kept in magnetic hot stirrer at 50-60°C for anhourand the colour gets changed from pale green to colourless which indicated the metal ion reduction. Upon the addition of curcumin extract (1 mmol),the colourless solutionin stantly turned yellow and the hot stirring was continued for about 2 hours.The reaction mixture colour was changed to yellowish brown and finally a permanent brown colour which implied that complete stabilized ZONP. The reaction pH and temperature were maintained in between 3 to 4 and 50-60°C respectively throughout the experiment. The mixture was centrifuged and washed well to obtain the pure ZONP. The super natant was decanted and dried in oven to use further analyses.
Synthesis of biofunctionalized zinc oxide nanoparticles (ZNSC)
In a typical synthesis of biofunctionalized zinc oxide nanoparticles,functionalization of salicyalalchitosan with zinc oxide nanoparticles was carried out under constant stirring and heating at 60°C. The above synthesized 1 mmol of ZONP was mixed with 1mmol of salicylalchitosan and kept on hot magnetic stirrer for an hour. The mixture color was turned to change from brown colour to dark brown which denoted the salicylalchitosan functionalized zinc oxide nanoparticles. The solution was centrifuged and the upper layer was removed and dried for furtheranalyses.
Biological Assay
The antibacterial activity of the samples were tested against two gram positive bacteria (Staphylococcus aureus and Bacillus subtilis), two gram negative bacteria (Escherichia coli and Salmonella typhi) and antifungal activity was carried out against four funguses (Candida albicans, Curvularia lunata, Aspergillus niger and Trichophyton simii).
The antibacterial activity of samples were measured by disc diffusion method (18). In this method, the stock cultures were incubated in nutrient agar and transferred to Muller-Hinton broth (MHB) contained test tube for bacteria that were incubated for 24 hours at 37°C. The cultures were diluted with fresh Muller-Hinton broth to obtain 2.0×106 CFU/ml for bacteria. The Muller Hinton Agar (MHA) plates were prepared by pouring 15 ml of molten media into sterile petri plates. The sample was loaded on the surface of the cultured agar plates and incubated at 37°C for 24 hours. The inhibition zones observed around the disc were measured.
The antifungal activity of samples were measured by agar well diffusion method (19), the fungal strains were suspended in sabouraud’s dextrose broth for 6 hours to give concentration 105CFU/ml and then inoculated with the culture medium. A total of 8 mm diameter wells were punched into the agar and filled with the sample and solvent blanks (hydro alcohol and hexane). Standard antibiotic, Fluconazole (concentration 1 mg/ml) was used as positive control and fungal plates were incubated at 37°C for 72 hours. The diameters of zone of inhibition observed were measured.
Results and Discussion
The UV-Visible absorption spectra of the samples were measured on a Shimadzu UV-Vis V-530A spectrophotometer in the range of 300 to 900 nm.Figure 1 and 2 shows a UV spectrum of non-functionalized and biofunctionalized zinc oxide nanoparticles respectively. The UV spectra of ZONP exhibited a peak at 300 nm which belongs to the absorption of zinc oxide nanoparticles (Figure 1). In the case of ZNSC, the same zinc oxide nanoparticles absorption peak was observed at 301 nm and next to this, there was a broaden peak has been observed at 392 nm which could be due to the functionalization of salicylalchitosan with zinc oxide nanoparticles (Figure 2). The other peak observed in lower wavelength in both formulations at around 225 nm could be due to the presence of aggregation of ZONP during the nanoparticle synthesis.
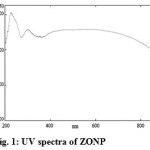 |
Figure 1: UV spectra of ZONP Click here to View figure |
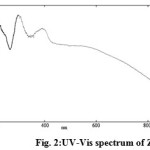 |
Figure 2: UV-Vis spectrum of ZNSC Click here to View figure |
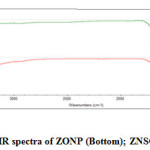 |
Figure 3: IR spectra of ZONP (Bottom); ZNSC (Top) |
FT-IR spectra analysis was recorded on a Jasso FT-IR/4100 spectrophotometer with 4 cm-1 resolution in the range of 4000 to 400 cm-1.Figure 3 shows the FT-IR spectrum of zinc oxide nanoparticles (Bottom) and functionalized nanoparticles (Top). ZONP showed the following important peaks at different regions, 3389 cm-1(ph-OH of curcumin), 2926 cm-1 (C-H stretching of water or ethanol), 1704cm-1(C=O stretching of curcumin), 1393cm-1(C=C stretching of curcumin), 1000-1250cm-1(C-O stretching-3 bands).In the case of ZNSC, the observed important peaks are at 3390 cm-1 (O-H polymeric stretching of chitosan), 1703 cm-1 (C=O stretching of SC), 1574 cm-1 and 1557 cm-1(C=C aromatic ring stretching), 1385 cm-1 (O-H stretching of ph-OHCHO coordinated with chitosan), 1179 cm-1 (C-N stretching of tertiary amine of chitosan), 1032 cm-1 (C-N stretching of CH=N present in SC),800-900 cm-1(C-O band).
Scanning electron microscopy (SEM) images were recorded by using JEOL Model JSM – 6390LV scanning electron microscope.The SEM images of ZONP and ZNSC were shown in Figure 4a and 4b respectively. It can be view that ZONP shown rod and spherical shaped crystals morphology of material (Figure 4a). In the case of ZNSC, the image exhibited slightly agglomeratedand dispersed spherical shaped crystals morphology due to the nanoparticles oxidation (Figure 4b).
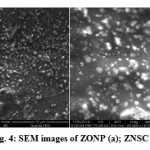 |
Figure 4: SEM images of ZONP (a); ZNSC (b) |
High resolution transmission electron microscopy (HRTEM) was carried out using a 300 KV JEOL-3011 instrument to determine the size and morphological changes.Figure 5a and 5b shows the TEM images of the non-functionalized and salicylalchitosan functionalized zinc oxide nanoparticles respectively. ZONP are slightly agglomerated and average crystallite size of particles is in the range of 23-27 nm with different shaped morphology (Figure 5a). ZNSCexhibits moderately dispersed spherical morphology and the average crystallite size of particles are in the range of46-50 nm. The size increment may due to the functionalization of salicylalchitosan with ZONP(Figure 5b).
 |
Figure 5: TEM images of ZONP (a); ZNSC (b) |
Antibacterial Activity
The antibacterial activity of biologically produced nanoparticles were tested on different bacteria Staphylococcus aureus,Bacillus subtilis, Escherichia coli and Salmonella typhi.The inhibition tests were compared to standard drug, Chloramphenicol. The results are reported in Table 1.
Table 1: Antibacterial activity evaluation
|
Bacterial Species |
Zone of inhibition diameter (mm sample-1) |
||
|
Standard drug (C) |
Zinc oxide nanoparticles (ZONP) |
Biofunctionalized zinc oxide nanoparticles (ZNSC) |
|
|
S. aureus |
13 |
14 |
20 |
|
B.subtilis |
15 |
15 |
16 |
|
E. coli |
11 |
08 |
06 |
|
S. typhi |
14 |
13 |
13 |
From Table 1, the results of biofunctionalized zinc oxide nanoparticles shown better antibacterial activity than zinc oxide nanoparticles. Non-functionalized ZONPshown reasonable inhibition activity against S.aureus, B. subtilis and S. typhi species. But ZNSC displayed an appreciable inhibition activity than ZONP against the above species. It showed similar activity to ZONP against S. typhi and lower activity against E.coli only. Especially, it showed higher inhibition zone results than standard drug,Chloramphenicol against S.aureus and B.subtilis strains.The difference observed in the activity enhancement is due to the biomolecule functionalization which increased the antimicrobial efficiency to the nanoparticles.
Antifungal Activity
ZONP and ZNSC were determined for their antifungal activity against four fungal strains Candida albicans, Curvularia lunata, Aspergillus niger and Trichophyton simii and their activity was compared with standard antifungal drug Fluconazole. The results were shown in Table 2.
Table 2: Antifungal activity evaluation
|
Fungal Species |
Zone of inhibition diameter (mm sample-1) |
||
|
Standard drug (C) |
Zinc oxide nanoparticles (ZONP) |
Biofunctionalized zinc oxide nanoparticles (ZNSC) |
|
|
C.albicans |
16 |
12 13 17 15 |
19 |
|
C.lunata |
15 |
13 |
|
|
A.niger |
15 |
17 |
|
|
T.simii |
13 |
20 |
|
From Table 2, the results revealed that biofunctionalized zinc oxide nanoparticles observed higher antifungal activity than non-functionalized zinc oxide nanoparticles. ZONP showed similar activity to ZNSC against A. niger and C.lunata fungal strains, however, the inhibition zone results observed by ZNSC were higher than ZONP as well as standard drug, Fluconazole against C.albicans and T.simiifungal species and the overall antifungal activity of ZNSC were more appreciable. Therefore, functionalization of salicylalchitosan is the important factor in the result of increment.
In summary, this investigation has been carried out to synthesize salicylalchitosan functionalized zinc oxide nanoparticles by interacting zinc oxide nanoparticles with salicylalchitosan.The antimicrobial activity studies expressed that biofunctionalized nanoparticles were showed better activity than the non-functionalized nanoparticles against S.aureus, B.subtilis,C.albicans and T.simii.The morphology studies were shown that ZONPsize was about 23-27 nm and ZNSC was in the range of 46-50 nm. This may due to the biofunctionalization, however, there was anincrementof antimicrobial activity only observed in the antimicrobial tests.Particularly, ZNSC shown higher activity than the standard drug against S.aureus, B. subtilis, C. albicans and T. simii. Therefore, our report reveals that biofunctionalization of salicylalchitosan increases the antimicrobial activity of zinc oxide nanoparticles which gives the new idea in the biomaterial utilized nanoparticle preparation.
Acknowledgements
We thank AMET University, Chennai, India for their support to do this work. We gratefully acknowledge Advanced Instrumentation Research Facility, Jawaharlal Nehru University, New Delhi for TEM analysis, Nanotechnology Research Centre, SRM University, Chennai for SEM analysis.
References
- Kavitha, K.S.; Syed Baker.; Rakshith, D.; Kavitha, H.U.; Yashwantha Rao, H.C.; Harini, B.P.;Satish, S. International Research Journal of Biological Sciences. 2013, 2, 66-76.
- Challa Kumar, S.S.R. Biological and pharmaceutical nanomaterials,Vol. II.; Wiley–VCH Verlag GmbH Co. KGaA. Weinheim, (2005).
- Goesmann, H.;Feldmann, C.Angew. Chem. Int. Ed.2010, 49, 1362–1395.
CrossRef - Katz, E.;Willner, I. Angew. Chem. Int. Ed.2004, 43, 6042–6108.
CrossRef - Kim, S.J.; Shin, S.R.; Lee, Y.M.; Kim, S.I. J. Appl. Polym. Sci. 2003, 87, 2011–2015.
CrossRef - Lei, C. X.; Hu, S. Q.; Shen, G. L.; Yu, R.Q. Talanta,2003, 59, 981–993.
CrossRef - Stoimenov, P. K.; Klinger, R. L.; Marchin, G.L.; Glebunde, K. Lang. 2002,18, 6679-6686.
CrossRef - Narayanan, R.; and El-Sayed, M. A. J. Am. Chem. Soc.2003,125, 8340-8347.
CrossRef - Brayner, R.;Ferrari-Iliou, R.; Brivois, N.; Djediat, S. Nano Lett.2006, 6, 866–870.
CrossRef - Gajjar, P.;Pettee, B.; Britt, D. W.; Huang, W.; Johnson, W.P.; Anderson, A.J.Journal of Biological Engineering. 2009, 3, 1-13.
CrossRef - Jayandran, M.; Muhamed haneefa, M.Chem. Sci. Rev. Lett.2014, 3, 1050-1059.
- Limer, J.L.; and Speirs, V. Breast Cancer Res. 2004,6, 119-127.
CrossRef - Bandele, O. J.; and Osheroff, N.Chem Res Toxicol. 2008,21, 936-43.
CrossRef - Charles J. Brine.;Paul R. Austin.Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 1981, 70,173–178.
- Muzzarelli, R. A. A.; Jeuniaux, C.; Gooday, G. W.(Eds). Chitin in Nature and Technology, Plenum. New York, USA, (1986).
CrossRef - Bauer, R. W.; Kirby, M. D.; Sherris, J. C.; Turck M. Am. J. Clin. Pathol.1966, 45, 493-496.
CrossRef - Reem K. Farag.; and Riham R. Mohamed. Molecules,2013, 18, 190-203.
- Jeong, S.; Woo, K.; Kim, D.; Lim, S.; Kim, J. S.; Adv Funct Mater. 2008, 18, 679–686.
CrossRef - Almeida Gomes, B. P.; Ferraz, C. C.; Vianna, M. E.Braz. Dent. J.2002,13, 155-161.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









