Development of the method of obtaining donor-acceptor complexes of titanium tetrachloride as a precursor of oxide materials based on titanium oxide and silicon matrix for the catalytically active nanoparticles of platinum group metals
A. M. Nemeryuk and M. M Lylina
Federal State Unitary Enterprise ”State Research Institute of Chemical Reagents and Pure Chemical Substances”(FSUE “IREA”), 107076, Russia, Moscow, Bogorodskiy val, 3
DOI : http://dx.doi.org/10.13005/ojc/310478
Article Received on :
Article Accepted on :
Article Published : 10 Dec 2015
The method of preparation of the complex of titanium tetrachloride with dimethylformamide and reactivity when reacted with an alcohol and salts of hydrazine described. Obtained multinuclear complexes containing titanium and palladium as potential precursors of catalytically active materials.
KEYWORDS:Nanoparticles of platinum group metals; titanium dioxide; silica; titanium tetrachloride; non-aqueous media
Download this article as:| Copy the following to cite this article: Nemeryuk A. M, Lylina M. M. Development of the method of obtaining donor-acceptor complexes of titanium tetrachloride as a precursor of oxide materials based on titanium oxide and silicon matrix for the catalytically active nanoparticles of platinum group metals |
| Copy the following to cite this URL: Nemeryuk A. M, Lylina M. M. Structural studies of UHMWPE composite materials containing nanoparticles of titanium, zirconium and hafnium oxides. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13234 |
Introduction
As the matrices for applying the catalytically active particles of platinum group metals conventionally used in catalytic materials used for reducing the environmental impact of exhaust emissions of internal combustion engines (ICE) are used metal oxides having sufficiently high thermal stability under operating conditions. The most commonly used aluminum oxide containing various additives that affect the catalytic properties of the material and to effectively neutralize the exhaust gases with different compositions, reflecting a change in the chemical composition of the exhaust gas, depending on the operating mode of the internal combustion engine. Thus, the oxygen content varies greatly, and especially – products of incomplete combustion of hydrocarbons contained in the fuel and engine oil also significantly changes depending on the engine operating conditions susceptible to concentrations of carbon monoxide and nitrogen oxides. Traditionally, in the catalytically active material used for neutralization of exhaust gases of ICE admix alkaline earth metal oxides, e.g., barium, and lanthanum elements of subgroup – usually used ceria. Less commonly used other oxides of elements, such as – that silicon, zirconium, and others. Based on the high catalytic activity of titanium dioxide, and also considering the heat resistance of this compound were carried work aimed at developing a method of preparing an oxide-based systems of titanium oxide and silicon suitable for producing a catalytically active material containing a platinum group metal nanoparticles. In developing the method with one of the conditions was to ensure the possibility of technological processes of oxide materials with regard to the compatibility of these processes with the methods used to produce nanoparticles of platinum group metals, which allows the full range of technological operations without the need for additional procedures to intermediate isolation, purification of individual components catalytically active materials designed to reduce emissions engine. As it has been tasked to develop a method to create oxide materials containing firmly bound nanoparticles of platinum group metals, which is necessary for long-term preservation of the catalytic activity and stability. One way of solving the problems is the use of methods of preparation of oxide systems in non-aqueous media using soluble in aprotic organic solvents, of compounds of metals and organic substances – oxygen donors. Preparation oxides such conditions allows fine regulation of the composition of the resulting materials, introduced therein certain additives, for example, precursors of silica, and obtain nanoparticles of platinum metals simultaneously to yield oxides or directly after their formation in the reaction medium, thereby providing a strong bond nanoparticles of platinum metals and particles of oxide systems as possible to avoid the agglomeration of nanoparticles of platinum metals.
Since as a template for the catalytically active material designed to reduce the environmental impact of emissions into the environment by engine exhaust gas amount characteristics is selected to titanium dioxide, the processes of the formation of this compound by reacting titanium tetrachloride with alcohols in non-aqueous media. Along with the interaction of titanium tetrachloride and alcohols and other known methods of preparing titanium dioxide in in non-aqueous media comprising the reaction of titanium alkoxides of alcohols and ketones, as well as interaction with titanium tetrachloride ethers. These methods have been rejected as requiring prior preparation of the starting compounds is not the product of large-scale production, which is not optimal for further scaling developed techniques or specific hardware design, including the use of vessels operating under positive pressure.
Despite the fact that known and widely used methods for the preparation of titanium dioxide based on the thermal-oxidative process interaction of titanium tetrachloride with oxygen hydrolytic aqueous methods, decomposition of salts of carboxylic acids, we have chosen as a promising method of producing titanium dioxide by alcoholysis of titanium tetrachloride alcohols in non-aqueous media. Using this approach allows to obtain titanium dioxide particles of various shapes and sizes depending on the conditions of interaction. This method is compatible with the costs associated with obtaining complex okidnyh systems, including those containing palladium or other platinum group metal compounds which are suitable for the preparation of metallic nanoparticles of metals, typically sparingly soluble in aqueous media, but easily soluble in organic solvents.
The first method of producing titanium dioxide by reacting titanium tetrachloride with benzyl alcohol was described in the papers relating to the preparation of nanoparticles of titanium dioxide and other elements [1 – 6]. When scaling method and the increasing concentration of the starting compounds were detected phenomenon not previously noted by the authors of works devoted to the production of oxide nanoparticles in this way.
In the course of numerous experiments on the interaction of titanium tetrachloride with benzyl alcohol in various conditions a significant amount of resinous organic compounds contaminating titanium dioxide and causing the need for further heat treatment of the products obtained by this method for the removal of organic impurities. As noted the influence of process conditions on the size and shape morphology of titanium dioxide particles. In order to reduce the content of organic impurities which are polymerization products formed by reacting titanium tetrachloride with benzyl alcohol benzylchloride steps were taken to reduce the catalytic activity of titanium tetrachloride, including by chelation. It is known that titanium tetrachloride is a compound belonging to the class of Lewis acids, such compounds are capable of catalyzing many processes, including leading to the production of polymer products contaminating the desired final product – the oxide-based oxides of titanium and silicon. To reduce the catalytic activity of titanium tetrachloride as Lewis acids were taken to produce the complexes of this compound with an electronically redundant compounds.
Based on information that the titanium tetrachloride is the Lewis acid and is capable of forming adducts with an electronically redundant compounds [7-13] synthesis was undertaken compound of titanium tetrachloride and DMF.
Results and Discussion
Synthesis of the adduct of titanium tetrachloride and DMF
A solution of titanium tetrachloride in carbon tetrachloride was added dropwise with vigorous stirring to a solution of dimethylformamide in excess of the same solvent. Immediately observed the formation of yellow crystals. The product was filtered, washed with carbon tetrachloride and dried in vacuum to constant weight.
According to the analysis: Ti 14,20 (14,26) Cl 41,78 (42,22) N 8,27 (8,34) 21,44 (21,42) H 4,50 (4,20) Ti / Cl 3,97 (4,00)%. In parentheses are estimates. The molecular weight ebulioskopicheskim method in methylene chloride: 340 ± 15, which corresponds to the calculated 335.9.
The product complies with gross formula TiCl4 2 DMF, is a yellow crystalline solid with m.p. 220-225°C, is highly resistant to hydrolysis.
We have received a sample of the compound and burned the IR – spectrum of Fig. 1.
 |
Figure 1: IR – spectrum of the product obtained in the synthesis of the adduct of titanium tetrachloride and DMF Click here to View figure |
Next, a series of experiments. It was carried out by reacting with t-butyl alcohol to yield a low-melting, but white crystalline product IR – spectrum below Fig. 2.
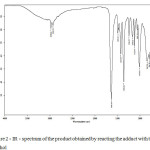 |
Figure 2: IR – spectrum of the product obtained by reacting the adduct with t-butyl alcohol Click here to View figure |
Reacting the adduct of titanium tetrachloride with t-butanol
In a round bottom flask equipped with a magnetic stirrer and reflux loaded 1.0 g of adduct of titanium tetrachloride and DMF. Was added 20 ml of t-butanol and heated with stirring at reflux temperature for 6 hours. Initially yellow color of the solution gradually turned into colorless. The reaction mixture was concentrated in vacuum using a rotary vacuum evaporator and then heated in vacuum to constant weight.
It was studied the interaction with palladium chloride – also takes place the formation of the complex, painted in a light pink color. Properties of the resulting complex are promising from the viewpoint of obtaining the potential of the catalytically active materials based on oxide matrix containing nanoparticles of platinum metals.
In both aqueous and non-aqueous media, these compounds are unstable in the presence of a base and immediately hydrolyzed with release of hydrated titanium oxide (not a hydrated or – if the interaction is carried out in non-aqueous media). If the base is used hydrazine hydrate, the first observed as loss of volume of white precipitate and a dark precipitate upon heating (which we associate with the formation of particles of palladium metal). To eliminate the influence of the basic properties of hydrazine hydrate, we carried out experiments on the interaction of chloride adduct of hydrazine. The complex obtained has a bluish or blue-violet depending on the concentration. IR – spectrum of the product is shown in Fig. 3.
Reacting the adduct with DMF titanium tetrachloride with hydrazine hydrochloride
In a round bottom flask equipped with a magnetic stirrer and reflux condenser was charged with 1.0 g of the adduct of titanium tetrachloride and DMF, and 0.5 g of hydrazine hydrochloride. Was added 20 ml of DMF and heated with stirring at 60-80°C the solvent for 6 hours. Initially yellow color of the solution gradually moved into the blue. The reaction mixture was concentrated in vacuo using a rotary vacuum evaporator and then heated in vacuum to constant weight.
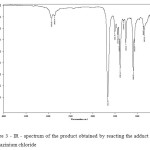 |
Figure 3: IR – spectrum of the product obtained by reacting the adduct with hydrazinium chloride Click here to View figure |
Also, we investigated the complex of titanium tetrachloride with DMSO. Similarly, the complex of titanium tetrachloride with DMSO is – yellow relatively low melting point (less than 100°C) substance is significantly more unstable to hydrolysis. It is also forms complexes with salts of palladium, but their appearance and properties vastly different. Thus the reaction with hydrazine hydrate white precipitate hydrated titanium oxide, whereas even the browning by heating is not observed. However, the treatment of this sludge ultrasonically observed darkening is apparently due to the formation of metallic palladium.
We also had a collaboration adduct of titanium tetrachloride and DMF with benzyl alcohol and palladium salts. Again, the resulting complex is characterized by increased resistance. If benzyl alcohol restores palladium salt even at room temperature, then the required heating to a temperature of 200°C, and simultaneously formed and the metallic palladium, and titanium oxide – evolution was observed gray powder. Analysis of the resulting product indicated the palladium content of 1.3-1.5%.
It seems that the structure of the resulting complex of titanium tetrachloride and DMF can be expressed by the formula (Fig. 4)
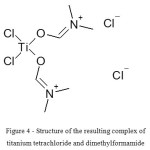 |
Figure 4: Structure of the resulting complex of titanium tetrachloride and dimethylformamide Click here to View figure |
Whereas in the case of complexes of the above compounds with palladium salts can act as counterion tetrachloropalladate.
Given the presence of absorption bands near 1636 cm-1 (this band titanium-chloro) in a compound with tert-butanol, it is assumed that the structure can be expressed by the formula shown in Fig. 5.
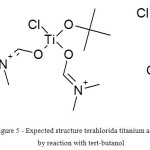 |
Figure 5: Expected structure terahlorida titanium adduct by reaction with tert-butanol Click here to View figure |
Conclusion
Preparation of the complexes reveals the feasibility of tasks one-pot syntheses of catalysts based on oxides of titanium, silicon and platinum group metals have been possible to carry out in a homogeneous reaction system, several chemical reactions leading to the formation of mixed oxide systems, including those containing metal particles of platinum group (Pd). Of particular interest is the ability of complexes of titanium tetrachloride and DMSO salts of palladium is reduced to metallic palladium only under the effect of ultrasound.
Acknowledgements
Applied researches are carried out with financial support of the state by the Russia Ministry of Education and Science under Grant Agreement No.14.624.21.0006 of September 8, 2014. (Unique identifier for Applied Scientific Researches (project) RFMEFI62414X0006).
References
- Rockenberger J., Scher E. C. Alivisatos A. P. J. Am. Chem. Soc. 1999, 121, 11595-11597
- Shim M., Guyot-Sionnest P. J. Am. Chem. Soc. 2001, 123, 11651-11654
- Joo J., Yu T., Young W. Kim, Park H. M., Wu F., Zhang J. Z., Hyeon T. J. Am. Chem. Soc. 2003, 125, 6553-6557
- Jun Y.-W., Casula M. F., Sim J.-H., Kim S. Y., Cheon J. Alivisatos A.P. J. Am. Chem. Soc. 2003, 125, 15981–15985
- Tang J.J., Fabbri, R. D., Robinson Y., Zhu I. P., Herman M. L. Chem Mater. 2004, 16, 1336-1342
- Niederberger M., Garnweitner G., Krumeich F., Nesper R., Cölfen H. Chem. Mater. 2004, 16, 1202-1208
- Ryndin Yu.A., Kogin Yu. N., Zaikovskii V.I., Jacob К., Thiele K.-H., Yemakov Yu. I. React. Kinet. Catal. Lett. 1985, 29(2), 395-402
- Ryndin Yu. H., Hogin Yu. K., Zverev Yu. B., Chernyaev N. P., Abdikova P.O., Yermakov Yu. I. React. Kinet. Catal. Lett. 1986, 31(1), 151-158
- Ryndin Yu. A., Nogin Yu. H., Chuvilin A. L., Pashis A. V., Zverev Yu. B., Yermakov Yu. I. Appl. Catal. 1986, 26(2), 327-338
- Ryndin Yu. A., Nogin Yu.N., Yermakov Yu. I. React. Kinet. Catal. Lett. 1988, 36(2), 345-350
- Ucgln Yu. N., Ryndin Yu. A. Catal. Cone, and Appl.: 9th Nat. Syrop. Catal. New Delhi, 1988, OR 24/1-24/15
- Kochubey D. I., Feodorov Y. K., Williams C., Stenin U. V., Ryndin Yu. A., Zazaxraev K. I. Catal. Lett. 1990, 5(4-6), 349-352
- Bonsu P.O., Lü X., Xie J., Jiang D., Chen M., Wei X. Kinet. Mech. Catal. 2012, 107, 487–502

This work is licensed under a Creative Commons Attribution 4.0 International License.









