Synthesis of Some New Bischromones from Bischalcones
K. G. Huge1*, V. M. Gurav2 and S. V. Anurkar1
1Department of Chemistry, K.K.M. College, Manwath, Dist. Parbhani, Maharashtra, India. 2Department of Chemistry, Yeshwant Mahavidayala, Nanded, Maharashtra, India.
Article Received on :
Article Accepted on :
Article Published : 10 Jun 2013
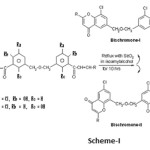
Some novel Di (6/8-chloro-8/6-Chromonyl) Methyl ethers i.e. Bischromones –I & II were synthesized by oxidative cyclisation of Di (5’-chloro-4’/2’-hydroxy-3’-chalconyl) methyl ethers i.e. Bischalcones- I & II and screened for antifungal activity.
KEYWORDS:Synthesis; Bischromones and Bischalcones
Download this article as:| Copy the following to cite this article: Huge K. G, Gurav V. M, Anurkar S. V. Synthesis of Some New Bischromones from Bischalcones. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Huge K. G, Gurav V. M, Anurkar S. V. Synthesis of Some New Bischromones from Bischalcones. Available from: http://www.orientjchem.org/?p=11971 |
Introduction
The Bischromones are used primarily in treating allergic asthma, exercise-induced asthma, sometimes in intrinsic asthma and also in the studies of various allergic lung diseases1. Chromolyn sodium (Intal) is used principally in the treatment of bronchial asthma2. It is the only biscromone drug now licensed by the Food & Drug Administration3. It is available as a powder and in capsule form. The Disodiumchromoglycate (DSCG/INTAL) and related compounds there of, including generally bischromones as anti allergenic, anti-PACVIS (Pathological cardiovascular ischemic state) agents4, and in the treatment of Bronchial asthma in children5. It also have an effect on the release of bistamine & degranulation of rat mast cells6. The Disodiumchromoglycate (DSCG) related compounds shows inhibition of homologous passive cutaneous anaphylaxis (PCA)7. Lomudal inhibits the bronchospam induced by the antigen & inhibits least partially the post exercise fall in FEV in children and adults8. A khelin like 7,7’-Glycerol bridged bischromone found anti anaphylacetic antivit9.
Use of bischromones in studies of various allergic lung diseases has provided researchers with valuable information about the body’s immune mechanisms. Therefore it was thought worthwhile to synthesize some new bischromones from bischalcones.
General Method
A mixture of Bischalcones-I/II (0.01 mole) and selenium oxide (0.08 mole) in isoamyl alcohol (50 ml) was refluxed for 10-12 hours. The reaction mixture was cooled and filtered to remove black precipitate of selenium formed during the reaction. Isoamyl alcohol from the filterate was removed by steam distillation and non-volatile residue thus obtained was extracted with hot benzene. Benzene layer was washed with aqueous NaHCO3 (10%) and then by distilled water to remove any acidic and water soluble impurities. The benzene was removed by evaporation and residue was crystallized from proper solvent (Scheme-I).
 |
Scheme 1: |
Experimental
The purity of the compounds was checked by TLC. Melting points of the compounds were determined in open capillary tubes and are incorrected. The IR spectra were recorded on Perkin-Elmer IR Infra cord in KBr disc. 1H-NMR spectra were recorded on a 300 MHz Brucker spectrometer using TMS as an internal standard. The titled compounds Bischromons-I & II i.e. Di (6/8- chloro-2-phenyl-8/6-Chromonyl) methyl ethers were synthesized by oxidative cyclisation of Bischalcones I & II i.e. Di (5’-chloro-4’/2’-hydroxy-3’-chalconyl) methyl ethers. These Bischalcones I & II on refluxing with SeO2 in isoamyl alcohol for 10 hrs., afforded Bis- Chromones- I & II (Table-I & II)
| [Table-I] : Di (8-chloro-6-Chromonyl) Methyl ethers i.e. Bischromones-I | ||||
| Sr. | R. | M.P.(oC) | Yield (%) | Analysis found (Calcd) Chlorine |
| Phenyl | 251 | 50 | 12.67 (12.79) | |
| o-Chlorophenyl | 162 | 65 | 22.42 (12.79) | |
| p-Chlorophenyl | 242 | 65 | 22.58 (22.75) | |
| o-Methyl phenyl | 233 | 70 | 12.48 (12.17) | |
| p-Methoxy phenyl | 225 | 72 | 11.33 (12.09) | |
| o-Hydroxy phenyl | 213 | 50 | 12.48 (12.09) | |
| m-Hydroxy phenyl | 217 | 68 | 12.47 (12.09) | |
| p-Hydroxy phenyl | 238 | 75 | 12.47 (12.09) | |
| a-Naphthyl | 266 | 70 | 10.62 (10.83) | |
| [Table-II] : Di (6-chloro-8-Chromonyl) Methyl ethers i.e. Bischromones-II | ||||
| Sr. | R. | M.P.(oC) | Yield (%) | Analysis found (Calcd) Chlorine |
| Phenyl | 226 | 50 | 12.67 (12.79) | |
| o-Chlorophenyl | 161 | 62 | 22.52 (22.75) | |
| p-Chlorophenyl | 216 | 60 | 22.38 (22.75) | |
| o-Methyl phenyl | 210 | 60 | 12.67 (12.17) | |
| p-Methoxy phenyl | 246 | 70 | 11.32 (12.09) | |
| a-Naphthyl | 261 | 75 | 10.62 (10.83) | |
Discussion of IR and 1H-NMR
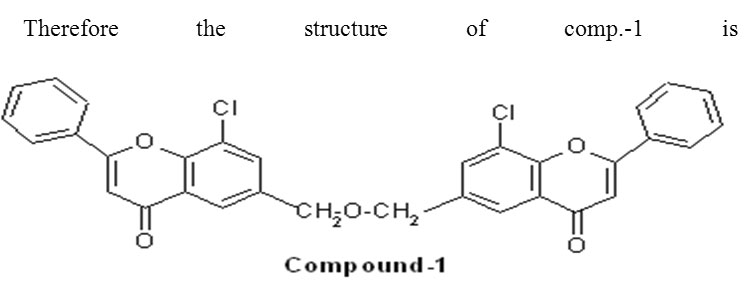
The representative member [comp.-1(Bischromons-I, from Table-I)] have shown following spectral data.
IR (KBr)
nmax cm-1:1635 (C=O), 1364-1361 (Pyrone-oxide vibration), 1563-1538 (ethylenic bond), 1100-1080 (asymmetrical (C-O-C stretch)
1H-NMR
(CDCl3, 300 MHz), dppm : 8.22 [d,2H,Jm=2.6 Hz,(H-5)], 8.07 [m,4H (H-2’,6’)], 7.60 [d,2H,Jm=2.6Hz,(H-7)],7.50 [m,6H, (H-3’,4’,5’], 6.78 [s,2H,(H-3)] ,4.03 [s,4H, (2HC-O-CH2)]
Therefore the structure of comp.-1 is Hence, the name of the compound is Di (8-chloro-2-phenyl-6-Chromonyl) Methyl ether.
Antifungal Activity
The antifungal screening of some of the representative compounds of the type I and II was carried out by dry weight method. In general they were found to be stimulatory against Penicillium Notatum at 100 ppm and 250 ppm concentrations.
Acknowledgement
The authors would like to thank Dr. N.V. Kalyankar, Principal, Yeshwant Mahavidyalaya, Nanded for providing laboratory facilities.
References
- Allergy Encyclopedia, (Dec 2008) and 3-Allergy Eneyclopedia (April 2007).
- Bernard A., Berman and Robert N.Ross, Clinical rev in allergy
- Immunology Vol I, No 1, , 105-121 (1983).
- Method of treating Ischemic states U S Patents-4584315.
- H Shioda, J Murano, K Mishima, Y Iikura, F Tanayka, M Izeki., Allergy, Vol 25, issue 2-3, 221-235 Pub On (2007).
- Orr T S, Hall D E, Gwilliam J M., Cox O S Life Sci, , Vol 10, issue 14, 805-812 (1971).
- Akhide koda, kenichi sakamoto and Yukiyoshi, Yana Gihara Jap Jour of pharma, Vol 30, No 4 , 437-447 (1980).
- J S G COX, R E C Altounyan. Respiration, Vol 27, Suppl (1970).
- Lowe W, Von maske P, Muller W, Arch Pharm (Weinbeim), 327, (4), 255-259 (1994).
- Adv In Heter Chem by Alan R katritcky, See IV, D P No 335.
- G Barker, G P Ellis and D Shaw Jur, Med Chem, Vol 16, No 1, P-87 (1973).
- Mohamad Yusuf, Rupesh kumar and S C Gupta ARKIVOC , (XV), 28-36 (2006).
- Suman Kalyan Panja, Sourav Maiti, Michael G B Drew & Candrakanta Bandyopadhyaya. Tetrahedron, Vol 65, issue 7, 1276-1280 (2009),.
- Lowe W, Rutjes T, Jour of hetero, Chem, Vol 32, No 1, 43-48 (1995).
- Tarun Ghosh, Satyajit Saha, Chandrakanta Bandyo Padhyaya, Thieme ejournals, Synthesis, , 1845-1849 (2005).
- Harnish H. Liebigs, Ann Chim, 8, 765 (1972).
- Glozman S M, Troitskaya V S, Vinokurav V G, Zagorevskii V A, Chem Hetero Cyc Comp, , 119, 5 (1969).
- Ermili A, Roma G, Balbi, A Farm, Ed Sci, , 29, 247 (1974).
- Hazard R, Wall E N Brit Patent, I, 324, 571 (1973).
- Chem Abst, 79, 126313 (1973).
- Dean F M fram Kham, D B Hatanm N , Hill A W ,J Chem. Soc., C 704 (1969).
- Bose D K Sen and Chankrawartik, An Boil Chem Expt Med 1945, 5 I, (1969).
- Sumnori U, Yoshinaris, Japan kokai, , 11, 882 (1974).
- Peter S Bevan, Gwoyann P Ellis and H Kerr Wilson, J Chem Soc, Perkin Trans 1, 2552-2556 (1981) .

This work is licensed under a Creative Commons Attribution 4.0 International License.









