Density Functional Study of Solvent and Substitute Effects on the Tautomerism of 3-Hydroxy-1,2,4-Oxadiazole Derivatives
Meisam Shabanian1*, Hassan Moghanian2, Mohsen Hajibeygi3 and Azin Mohamadi1
1Young Researchers Club, Arak Branch, Islamic Azad University, Arak (Iran). 2Department of Chemistry, Dezful Branch, Islamlic Azad University, Dezful (Iran). 3Department of Chemistry, Varamin Pishva Branch, Islamic Azad University, Varamin Pishva (Iran).
Article Received on :
Article Accepted on :
Article Published : 01 Jun 2012
Computational calculations at B3LYP/6-311++G(d,p) level were employed in the study of the predominant tautomeric forms of OH and NH 3-Hydroxy-1,2,4-Oxadiazole derivatives (5- NO2 , 5-CF3 , 5-F, 5-H, 5-CH3 , 5-OH, 5-NH2 ) in the gas phase and solution using PCM model. Then important molecular parameters, IR frequencies, NBO and dipole moment results in the gas phase and solvents were extracted. In the gas phase the stability of the tautomers relate to the nature of substituents. In the solution and with increase of polarity; NH isomers were more stable.
KEYWORDS:DFT study; 3-Hydroxy-1,2,4-Oxadiazole; Tautomerism; NBO analysis
Download this article as:| Copy the following to cite this article: Shabanian M, Moghanian H, Hajibeygi M, Mohamadi A. Density Functional Study of Solvent and Substitute Effects on the Tautomerism of 3-Hydroxy-1,2,4-Oxadiazole Derivatives. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Shabanian M, Moghanian H, Hajibeygi M, Mohamadi A. Density Functional Study of Solvent and Substitute Effects on the Tautomerism of 3-Hydroxy-1,2,4-Oxadiazole Derivatives. Available from: http://www.orientjchem.org/?p=11831 |
Introduction
Heterocyclic moieties such as 1,2,4-Oxadiazole rings can be found in a large number of compounds which display biological activity. 1,2,4-Oxadiazoles have been prepared by cycloadditions of nitrile oxides to amidoximes, treatment of acylated amidoximes with bases such as NaH or NaOEt at room temperature, or pyridine with heating, in solution phase and on solid support [1-3] and they are often used in drug discovery as hydrolysis-resisting bioisosteric replacements for ester or amide functionalities and has seen utility in producing potent, metabolically stable and bioavailable compounds in many research programs [4]. Numerous 1,2,4-oxadiazoles have been suggested as potential agonists for cortical muscarinic, 5-hydroxytryptamine receptors [5] and benzodiazepine [6]. They show activity as antirhinoviral agents, growth hormone secretagogues [7], anti-inflammatory agents [8] and antitumor agents [9]. They also inhibit the monoamine oxidase [10], human nuetrophil elastase [11], and human DNA topoisomerases [12]. Finally, tropane derivatives of 1,2,4-oxadiazoles display high affinity for the cocaine binding site of the dopamine transporter [13].
The prototropic tautomerisation and intramolecular proton transfer of the keto-enol reactions of heterocyclic systems with several basic centers, O, N and S atoms, are of great interest to medicinal and biochemical applications. Also, understanding of the relative stabilities of heterocyclic tautomers and any subsequent conversions between tautomeric forms is very vital for both structural chemists and biologists [14]. Along this line, relative stabilities of various tautomeric structures of five-, six- (oxo and thioxo groups in positions 2 and 4 respectively) and seven-membered heterocyclic rings (oxo and thioxo groups in positions 3 and 5 respectively) were investigated using both theoretical and experimental tools [15-19]. Both tools indicate that in these compounds the thioxo, dioxo or dithio tautomer is most stable.
The chemistry of 1,2,4-Oxadiazole is well known. One can find them as structural units in many compounds with applications in medicinal chemistry, photochemistry and biochemistry. Also their Tautomerism of five-membered heterocycles is important for pharmacy. The aim of this study is systematic investigation of substituent and solvent effect and its influence on tautomerism of the C5-substituted 3-hydroxy-1,2,4- Oxadiazoles.
Computational methods
All these calculations were carried out on a Pentium V personal computer by means of GAUSSIAN03 program package [20] and for our computations. First, all compound’s structures were drawn using Gauss View 03 [21]. To characterize all the optimized geometries the vibrational frequencies for all the conformers have been done at B3LYP levels. The stationary structures are confirmed by ascertaining that all ground states have only real frequencies. The tautomers were also optimized in solvents according to the polarisable continuum method of Tomasi and co-workers, which exploits the generating polyhedra procedure [22–25] to build the cavity in the polarisable continuum medium, where the solute is accommodated. Atomic charges in all the structures were obtained using the Natural Population Analysis (NPA) method within the Natural Bond Orbital (NBO) approach [26].
Results and discussion
Gas phase
Structures and numbering of 3-Hydroxy-1,2,4-Oxadiazole derivatives are depicted in Scheme 1 and the results of energy comparisons of two tautomers in the gas phase and different solvents are given in Table 1. In the gas phase when the subsistents change from withdrawing groups to electron donating groups NH forms are more stable than OH but we can see an exception in F subsistent because it can be a resonance donating group. The major difference between OH and NH form in gas phase was found for 5-triflouromethyl-3-Hydroxy-1,2,4-Oxadiazole with 3.43 kcal mol-1. The order of stability of OH tautomer over NH tautomer in the gas phase is 2 > 4 > 5 > 1 > 3 >7 > 6. This indicates that the stability of the tautomers relate to the nature of substituents.
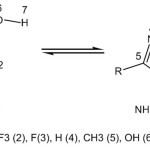 |
Scheme 1: Tautomeric forms of 3-Hydroxy-1,2,4-Oxadiazole derivatives and numbering of ring |
Table 1: Total energiesa at B3LYP/6-311++G** and relative energyb in the gas phase and in the solvents
|
Water (78.4) |
Methanol (33) |
Acetone (21.0) |
Benzene (2.2) |
Gas (1.0) |
Tautomer |
R |
|
-541.973824 |
-541.966951 |
-541.965842 |
-541.956099 |
-541.945050 |
OH |
|
|
-541.972321 |
-541.971552 |
-541.970812 |
-541.958288 |
-541.944369 |
NH |
NO2 |
|
-0.94 |
2.89 |
3.12 |
1.37 |
-0.43 |
E2-E1 |
|
|
-674.566580 |
-674.565785 |
-674.564906 |
-674.556349 |
-674.375081 |
OH |
|
|
-674.565601 |
-674.564874 |
-674.564184 |
-674.554038 |
-674.369607 |
NH |
CF3 |
|
-0.61 |
-0.57 |
-0.45 |
-1.45 |
-3.43 |
E2-E1 |
|
|
-436.694378 |
-436.693887 |
-436.693074 |
-436.684532 |
-436.674694 |
OH |
|
|
-436.697501 |
-436.696931 |
-436.696341 |
-436.686529 |
-436.675695 |
NH |
F |
|
1.96 |
1.91 |
2.05 |
1.25 |
0.63 |
E2-E1 |
|
|
-337.433041 |
-337.432529 |
-337.431625 |
-337.421989 |
-337.330047 |
OH |
|
|
-337.433800 |
-337.433184 |
-337.432563 |
-337.420934 |
-337.325698 |
NH |
H |
|
0.48 |
0.41 |
0.59 |
-0.66 |
-2.73 |
E2-E1 |
|
|
-376.769261 |
-376.768778 |
-376.767963 |
-376.759097 |
-376.748921 |
OH |
|
|
-376.771189 |
-376.770597 |
-376.770016 |
-376.759455 |
-376.747975 |
NH |
CH3 |
|
1.21 |
1.14 |
1.29 |
0.22 |
-0.59 |
E2-E1 |
|
|
-412.700043 |
-412.699249 |
-412.698113 |
-412.683330 |
-412.666751 |
OH |
|
|
-412.705225 |
-412.704369 |
-412.703563 |
-412.687833 |
-412.670469 |
NH |
OH |
|
3.25 |
3.21 |
3.42 |
2.82 |
2.33 |
E2-E1 |
|
|
-392.833277 |
-392.832549 |
-392.831559 |
-392.817801 |
-392.802168 |
OH |
|
|
-392.840130 |
-392.839214 |
-392.838355 |
-392.822540 |
-392.805367 |
NH |
NH2 |
|
4.30 |
4.18 |
4.26 |
2.97 |
2.01 |
E2-E1 |
aHartree
bRelative energy in kcal mol-1.
The optimized parameters of all structures are listed in Table 2. Important aspects of molecular structure can be observed in Table 2. The N2=C3 bond length, reported in the first row of table, lies in the range of 1.30–1.31 Å in OH tautomers. The N2=C3 bond length decreases with the increase of ring size, because of decreasing in ring strain. This indicates that N2=C3 bond length of OH form does not relate to the nature of substituents.
The N4=C5 bond length lies in the range of 1.28–1.31 Å in OH tautomers and 1.27-1.29 Å in NH tautomers. The N4=C5 bond length in both of tautomers increase in the present of electron donating groups. Next five rows of Table 2 consist of C3=O6, C3-O6, N2–C3, N2-H7 and O6-H7 bond lengths.
Table 2: Selected molecular parameters of optimized structure of 3-Hydroxy-1,3,4-Oxadiazole at B3LYP level of theorya
|
|
NO2 |
|
CF3 |
|
F |
|
H |
|
CH3 |
|
OH |
|
NH2 |
|
|
Parameter |
OH |
NH |
OH |
NH |
OH |
NH |
OH |
NH |
OH |
NH |
OH |
NH |
OH |
NH |
|
N2=C3 |
1.31 |
– |
1.31 |
– |
1.31 |
– |
1.31 |
– |
1.30 |
– |
1.31 |
– |
1.31 |
– |
|
N4=C5 |
1.28 |
1.27 |
1.29 |
1.27 |
1.29 |
1.27 |
1.29 |
1.27 |
1.30 |
1.28 |
1.30 |
1.28 |
1.31 |
1.29 |
|
C3=O6 |
– |
1.19 |
– |
1.19 |
– |
1.19 |
– |
1.19 |
– |
1.20 |
– |
1.19 |
– |
1.20 |
|
C3-O6 |
1.32 |
– |
1.33 |
– |
1.30 |
– |
1.33 |
– |
1.33 |
– |
1.33 |
– |
1.33 |
– |
|
N2–C3 |
– |
1.42 |
– |
1.42 |
– |
1.43 |
– |
1.42 |
– |
1.42 |
– |
1.44 |
– |
1.44 |
|
N2-H7 |
– |
1.01 |
– |
1.42 |
– |
1.02 |
– |
1.01 |
– |
1.01 |
– |
1.01 |
– |
1.02 |
|
O6-H7 |
0.96 |
– |
0.96 |
– |
0.96 |
– |
0.96 |
– |
0.96 |
– |
0.96 |
– |
0.96 |
– |
a All bond length have been reported in Å
The calculated dipole moments for the tautomers are presented in Table 3. It is notable that dipole moments significantly relate to the nature of substituents at the 5th position. In the both the tautomers, electron withdrawing derivatives have smaller dipole moments than electron releasing ones. This maybe explained by consideration of charge values on atoms of 1,2,4-Oxadiazole ring. It is well known that in OH and NH tautomers N4 atom carries the most negative charge. The OH isomer of 5-CF3 derivative has the least charge density on N4 but NH isomer of 5-NO2 derivative has the least charge density on N4. It is noticeable that the differences between dipole moments of OH and NH forms are related to nature of substituents. For example for methyl and amine derivatives difference between dipole moment of OH and NH is 1.34 and 1.37 D but for NO2 and CF3 the values are 0.757 and 0.968 D respectively.
Table 3: Calculated dipole moments of optimized tautomers (Debye)
|
Water (78.4)* |
Methanol (33.0)* |
Acetone (21.0)* |
Benzene (2.2)* |
Gas (1.0)* |
Tautomer |
R |
|
4.3906 |
3.9920 |
3.9590 |
3.3498 |
2.7590 |
OH |
NO2 |
|
5.4260 |
5.3721 |
5.3391 |
4.3546 |
3.5160 |
NH |
|
|
3.5376 |
3.5259 |
3.4912 |
2.9164 |
2.3711 |
OH |
CF3 |
|
5.3107 |
5.2141 |
5.1501 |
4.1871 |
3.3391 |
NH |
|
|
3.4452 |
3.4119 |
3.3677 |
2.8422 |
2.3386 |
OH |
F |
|
5.4055 |
5.3476 |
5.2948 |
4.3890 |
3.5533 |
NH |
|
|
4.1270 |
4.0989 |
4.0475 |
3.5240 |
2.9963 |
OH |
H |
|
6.6404 |
6.5903 |
6.4846 |
5.4288 |
4.4168 |
NH |
|
|
4.7659 |
4.7408 |
4.6970 |
4.2163 |
3.7299 |
OH |
CH3 |
|
7.1890 |
7.1337 |
7.0807 |
6.0389 |
5.0734 |
NH |
|
|
6.4356 |
6.3817 |
6.3138 |
5.3471 |
4.4198 |
OH |
OH |
|
5.6418 |
5.5866 |
5.5213 |
4.5138 |
4.4190 |
NH |
|
|
6.6273 |
6.5779 |
6.5200 |
5.5974 |
4.7272 |
OH |
NH2 |
|
9.2075 |
9.1202 |
9.0478 |
7.5955 |
6.1046 |
NH |
* relative dielectric constant
The calculated values NBO charges using the Natural Population Analysis (NPA) of optimized structures of 3-Hydroxy-1,2,4-Oxadiazole derivatives are listed in Table 4. As it was noticed previously, 3-Hydroxy-1,2,4-Oxadiazole’s nitrogen and oxygen atom at position 1, 2 or 4 carry the largest negative charge and these positions will most effectively interact with electrophiles. However N2 atoms of NH tautomers have higher charge compare with N2 atoms of OH tautomers. There is no uniform trend for the variation of charges to relate to the different substituents of 3-Hydroxy-1,2,4-Oxadiazole in the gas phase, Table 4.
Table 4: Calculated NBO charges on ring atoms of Maleic Hydrazide
|
(78.4) |
(33.0) |
(21.0) |
(2.2) |
(1.0) |
(78.4) |
(33.0) |
(21.0) |
(2.2) |
(1.0) |
ϵ= |
|
|
NH |
OH |
Atom |
R |
||||||||
|
-0.296 |
-0.297 |
-0.297 |
-0.310 |
-0.318 |
-0.294 |
-0.310 |
-0.310 |
-0.305 |
-0.303 |
O1 |
|
|
-0.314 |
-0.314 |
-0.314 |
-0.323 |
-0.326 |
-0.215 |
-0.235 |
-0.232 |
-0.208 |
-0.183 |
N2 |
|
|
0.758 |
0.758 |
0.757 |
0.753 |
0.745 |
0.659 |
0.661 |
0.660 |
0.647 |
0.633 |
C3 |
NO2 |
|
-0.511 |
-0.510 |
-0.508 |
-0.493 |
-0.479 |
-0.502 |
-0.526 |
-0.527 |
-0.532 |
-0.535 |
N4 |
|
|
0.695 |
0.693 |
0.692 |
0.681 |
0.665 |
0.672 |
0.684 |
0.683 |
0.678 |
0.673 |
C5 |
|
|
-0.305 |
-0.307 |
-0.308 |
-0.320 |
-0.329 |
-0.308 |
-0.307 |
-0.307 |
-0.301 |
-0.295 |
O1 |
|
|
-0.321 |
-0.322 |
-0.322 |
-0.328 |
-0.330 |
-0.238 |
-0.236 |
-0.234 |
-0.208 |
-0.181 |
N2 |
|
|
0.753 |
0.753 |
0.753 |
0.750 |
0.742 |
0.652 |
0.652 |
0.651 |
0.638 |
0.624 |
C3 |
CF3 |
|
-0.522 |
-0.520 |
-0.518 |
-0.502 |
-0.487 |
-0.515 |
-0.514 |
-0.514 |
-0.516 |
-0.516 |
N4 |
|
|
0.515 |
0.515 |
0.513 |
0.505 |
0.495 |
0.491 |
0.489 |
0.488 |
0.482 |
0.474 |
C5 |
|
|
-0.337 |
-0.338 |
-0.339 |
-0.350 |
-0.357 |
-0.342 |
-0.342 |
-0.341 |
-0.336 |
-0.332 |
O1 |
|
|
-0.340 |
-0.340 |
-0.340 |
-0.340 |
-0.338 |
-0.270 |
-0.269 |
-0.266 |
-0.237 |
-0.208 |
N2 |
|
|
0.763 |
0.763 |
0.763 |
0.758 |
0.750 |
0.662 |
0.661 |
0.660 |
0.647 |
0.633 |
C3 |
F |
|
-0.603 |
-0.602 |
-0.601 |
-0.582 |
-0.562 |
-0.596 |
-0.596 |
-0.596 |
-0.595 |
-0.591 |
N4 |
|
|
0.964 |
0.963 |
0.962 |
0.949 |
0.935 |
0.945 |
0.944 |
0.944 |
0.935 |
0.924 |
C5 |
|
|
-0.320 |
-0.320 |
-0.322 |
-0.333 |
-0.341 |
-0.327 |
-0.327 |
-0.326 |
-0.319 |
-0.315 |
O1 |
|
|
-0.337 |
-0.338 |
-0.339 |
-0.341 |
-0.341 |
-0.272 |
-0.270 |
-0.268 |
-0.236 |
-0.210 |
N2 |
|
|
0.748 |
0.748 |
0.749 |
0.746 |
0.741 |
0.645 |
0.645 |
0.644 |
0.633 |
0.622 |
C3 |
H |
|
-0.581 |
-0.579 |
-0.578 |
-0.552 |
-0.526 |
-0.574 |
-0.573 |
-0.574 |
-0.567 |
-0.562 |
N4 |
|
|
0.447 |
0.446 |
0.447 |
0.438 |
0.429 |
0.419 |
0.418 |
0.418 |
0.413 |
0.383 |
C5 |
|
|
-0.347 |
-0.347 |
-0.348 |
-0.360 |
-0.368 |
-0.347 |
-0.346 |
-0.346 |
-0.393 |
-0.333 |
O1 |
|
|
-0.338 |
-0.338 |
-0.338 |
-0.341 |
-0.340 |
-0.279 |
-0.277 |
-0.275 |
-0.244 |
-0.213 |
N2 |
|
|
0.756 |
0.755 |
0.755 |
0.753 |
0.748 |
0.651 |
0.651 |
0.650 |
0.638 |
0.626 |
C3 |
CH3 |
|
-0.592 |
-0.591 |
-0.589 |
-0.569 |
-0.551 |
-0.584 |
-0.584 |
-0.584 |
-0.581 |
-0.574 |
N4 |
|
|
0.633 |
0.632 |
0.631 |
0.619 |
0.607 |
0.605 |
0.605 |
0.604 |
0.595 |
0.583 |
C5 |
|
|
-0.357 |
-0.357 |
-0.357 |
-0.358 |
-0.358 |
-0.364 |
-0.363 |
-0.362 |
-0.348 |
-0.334 |
O1 |
|
|
-0.352 |
-0.352 |
-0.352 |
-0.348 |
-0.343 |
-0.298 |
-0.296 |
-0.293 |
-0.256 |
-0.221 |
N2 |
|
|
0.761 |
0.761 |
0.761 |
0.758 |
0.752 |
0.660 |
0.659 |
0.658 |
0.647 |
0.635 |
C3 |
OH |
|
-0.648 |
-0.647 |
-0.646 |
-0.630 |
-0.612 |
-0.638 |
-0.639 |
-0.639 |
-0.640 |
-0.637 |
N4 |
|
|
0.887 |
0.886 |
0.885 |
0.871 |
0.856 |
0.867 |
0.866 |
0.866 |
0.856 |
0.843 |
C5 |
|
|
-0.381 |
-0.381 |
-0.382 |
-0.389 |
-0.393 |
-0.382 |
-0.382 |
-0.381 |
-0.371 |
-0.363 |
O1 |
|
|
-0.361 |
-0.360 |
-0.360 |
-0.354 |
-0.346 |
-0.319 |
-0.317 |
-0.314 |
-0.273 |
-0.234 |
N2 |
|
|
0.759 |
0.759 |
0.759 |
0.757 |
0.753 |
0.659 |
0.658 |
0.658 |
0.648 |
0.637 |
C3 |
NH2 |
|
-0.663 |
-0.662 |
-0.660 |
-0.635 |
-0.606 |
-0.653 |
-0.653 |
-0.653 |
-0.642 |
-0.629 |
N4 |
|
|
0.758 |
0.757 |
0.756 |
0.747 |
0.737 |
0.739 |
0.738 |
0.738 |
0.732 |
0.724 |
C5 |
Five important vibrational frequencies of all structures are listed in Table 5. In the first row, N1–H7 frequency (this frequency only exists in tautomer NH). Next row of Table 5 consists of O6-H7. In three last rows, N2=C3, C3=O6 and N4=C5 frequencies are shown.
Table 5: Selected frequencies (in cm-1) of tautomers at B3LYP/6-311++G** level of theory in the gas phase
|
|
NO2 |
|
CF3 |
|
F |
|
H |
|
CH3 |
|
OH |
|
NH2 |
|
|
Bond |
OH |
NH |
OH |
NH |
OH |
NH |
OH |
NH |
OH |
NH |
OH |
NH |
OH |
NH |
|
N2–H7 |
– |
3572.8 |
– |
3576.3 |
– |
3554.7 |
– |
3579.8 |
– |
3571.4 |
– |
3791.0 |
– |
3540.7 |
|
O6–H7 |
3812.7 |
– |
3814.6 |
– |
3816.9 |
– |
3813.9 |
– |
3820.0 |
– |
3825.4 |
– |
3822.1 |
– |
|
N2=C3 |
1673.3 |
– |
1672.4 |
– |
1685.1 |
– |
1668.7 |
– |
1667.3 |
– |
1674.2 |
– |
1660.6 |
– |
|
C3=O6 |
– |
1867.0 |
– |
1857.4 |
– |
1870.6 |
– |
1842.9 |
– |
1838.7 |
– |
1853.8 |
– |
1845.3 |
|
N4=C5 |
1673.3 |
1676.1 |
1672.4 |
1692.4 |
1685.1 |
1700.9 |
1668.7 |
1629.2 |
1667.3 |
1667.7 |
1674.2 |
1668.8 |
1691.5 |
1701.2 |
Solvent effects
Solvent effects are relevant in tautomer stability phenomena, since polarity differences among tautomers can induce significant changes in their relative energies in solution. We decided to use of PCM/B3LYP calculations to analyze the solvent effects on tautomerism of 3-Hydroxy-1,2,4-Oxadiazole derivatives. It is important to stress that the PCM model does not consider the presence of explicit solvent molecules; hence specific solute-solvent interactions are not described and the calculated solvation effects arise only from mutual solute–solvent electrostatic polarization. The data presented in Table 1 show that polar solvents increase the stability of all 3-Hydroxy-1,2,4-Oxadiazole in compare to gas phase. The difference between the total energies of OH and NH with electron withdrawing and electron donating substituents do not show a regular trend when changing from gas phase to most polar solvents (water). Finally, the charges’s sign is reversed with NH tautomer becoming more stable than OH isomer except for NO2 group in water. For example, the differences of energy between OH and NH form of 5-methyl tetrazole are -0.59 and +1.21 kcal mol-1 in the gas phase and in water, respectively.
The solvent interactions have pronounced effect on the order of stability of the tautomers in the gas phase. For example, in benzene with low dielectric constant, order of stabilities of OH over NH form are 2 > 4 > 5 > 3 > 1 >6 > 7 but in water the order is 1 > 2 > 4 > 5 > 3 > 6 > 7 (compounds 1 and 2, OH tautomer are more stable). The solvent represented by a polarizable continuum is found show significant effect on the dipole moments of the individual solute conformers. The dipole moments (µ) increases by changing the gas phase to the solution as well as by increasing the solvent polarity. The most significant variations being obtained in NH tautomer of compound 7 with 3.1029 D, Table 3.
In addition, for the electron withdrawing substituents, the differences between the dipole moments in solvents (with high dielectric constants) and the gas phase are smaller than the electron donating groups. Therefore, the increase stability of NH tautomers with electron releasing groups in polar solvents could be related to the increase of dipole moments of NH forms over OH forms. The charge distributions of dipolar compounds are often altered significantly in the presence of a solvent reaction field [27]. We have examined the charge distribution of tautomers in the solvent as well as gas phase by using calculated NBO charges. The charge distribution in solvents with increase of polarity differently varies for any atoms. For example, a regular decrease of negative charge was found for N4 atom in OH forms of compound 1 when passing from gas phase to more polar solvent water, but for the NH form an increase of negative charge was obtained. In N2 position the negative charge of NH isomers in compound 1 and 2 from gas phase to polar solvents decrease but in compound 6 and 7 increased drastically. When passing from gas phase to polar solvents a regular increase of negative charge in the N4 position in NH tautomer was found. Charge on Carbon atom does not show any relationship to the nature of substituent, however, with the increase of polarity a regular increase of positive charge was observed for OH and NH derivatives. For example, in 5-NO2 derivative in NH tautomer the charge on carbon was found 0.665, 0.681, 0.692, 0.693, and 0.695 for the gas phase, benzene, Acetone, methanol and water, respectively.
Conclusions
In this work, DFT calculation has been applied to study of tautomerism in 3-Hydroxy-1,2,4-Oxadiazole with deferent subsistents in position 5 in the gas phase and four solvent. The following points emerge from the present study:
1. In the gas phase the stability of the tautomers relate to the nature of substituents in compound 6 and 7 with strong electron releasing groups the NH was very more stable than OH tautomer. In the solution and with increase of polarity; NH isomers were more stable. With increase of polarity total energy of all compounds were more negative.
2. The dipole moments of all compounds are affected by solvent. With increase of the polarity of solvents the dipole moments of OH and NH tautomers were increased.
3. The charges on all five positions were affected by substituents and solvents. The net charge on compounds with electron with drawing substituents are less than electron releasing groups. In addition with increase of dielectric constant a regular variation was found.
References
- Kayukova L. A., Praliev K. D., Zhumadildaeva I. S. and Klepikova S. G., Chem. Heterocycl. Comp., 35, 630 (1999).
- Hebert N., Hannah A. L. and Sutton S. C., Tetrahedron. Lett., 40, 8547 (1999).
- Gangloff A. R., Litvak J., Shelton E. J., Sperandio D., Wang V. R. and Rice K. D., Tetrahedron. Lett., 42, 1441 (2001).
- KumarD., Patel D., Chavers A. K., Chang K. H. and Shah K., Eur. J. Med. Chem., 46, 3085 (2011).
- Katritzky A. R., Shestopalov A. A and Suzuki K., ARKIVOC., vii, 36. (2005)
- Adib M., Jahromi A. H., Tavoosi N., Mahdavi M. and Bijanzadeh H. R., Tetrahedron. Lett., 47, 2965 (2006).
- Kaboudin B. and Saadati F., Tetrahedron Lett., 48, 2829 (2007).
- Farooqui M., Rajesh Bora R. and Patil C. R., Eur. J. Med. Chem., 44, 794 (2009).
- Kemnitzer W., Kuemmerle J., Zhang H. Z., Kasibhatla S., Tseng B., Drewe J. and Cai S. X., Bioorg. Med. Chem. Lett. 19, 4410 (2009).
- Kumar D., Patel G., Johnson E. O. and Shah K., Bioorg. Med. Chem. Lett., 19, 2739 (2009).
- Ohmoto K., Yamamoto T., Horiuchi T., Imanishi H., Odagaki Y., Kawabata K., Sekioka T., Hirota Y., Matsuoka S., Nakai H. and Toda M., J. Med. Chem., 43, 4927 (2000).
- Rudolph J., Theis H., Hanke R., Endermann R., Johannsen L. and Geschke F. U., J. Med. Chem., 44, 619 (2001).
- Adhav G. R., Shaikh M. U., Kale R. P., Ghawalkar A. R. and Gill C. H., J. Heterocycl. Chem., 46, 980 (2009).
- Minkin V. I., Garnovski A. D., Elguero J., Katritzky A. R. and Denisko O., Adv. Heterocycl. Chem., 38, 1110 (2002).
- Tahmassebi D., J. Mol. Struct. (THEOCHEM)., 638, 11 (2003).
- Shabanian M, Moghanian H, Hajibeygi M and Mohamadi A. E-J. Chem., 9, 107 (2012).
- Santos M., Junior M., Oliviera S., da Silva J., Lima M., Galdino S. and Pitta I., J. Mol. Struct. (THEOCHEM)., 715, 191 (2005).
- Yu F. L., Schwalbe C. H. And Watkin D., Acta. Cryst. C., 60, 714 (2004).
- Safiz S. and Abu-Awwad F. M., E-J. Chem., 5, 884 (2008).
- Frisch M. J., Trucks G. W. and Schlegel H. B., et al., Gaussian 03, Revision B.03, Gaussian, Inc. Pittsburgh PA (2003).
- Dennington R., Keith T., Millam J., Eppinnett K., Hovell W. L. and Gilliland R., GaussView, Version 309., Semichem, Inc, Shawnee Mission, KS (2003).
- Miertus S., Scrocco E. and Tomasi J., J. Chem. Phys., 55, 117 (1981).
- Cances M. T., Mennucci V. and Tomasi J., J. Chem. Phys., 107, 3032 (1997).
- Cossi M., Barone V., Mennucci B. and Tomasi J., Chem. Phys. Lett., 286, 253 (1998).
- Barone V., Cossi M. and Tomasi J., J. Comp. Chem., 19, 404 (1998).
- Najafi Chermahini A., Nasr-Esfahani M., Dalirnasab Z., Abdol Dabbagh H. and Teimouri A., J. Mol. Struct, (THEOCHEM)., 820, 7 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.









