Photodegradation of Dye Pollutant on Ag/ZnO Nanocatalyst under UV-irradiation
Masoumeh Tabatabaee* and Seyed Abolfazl Mirrahimi
Department of Chemistry, Islamic Azad University- Yazd Beranch, Yazd, Iran.
Article Received on :
Article Accepted on :
Article Published : 05 Mar 2011
Ag/ZnO heterostructure nanocatalyst was successfully prepared. The degradation of reactive blue 49, commonly used as a textile dye, can be photocatalysed by Ag/ZnO. Photodegradation efficiency was small when the photolysis was carried out in the absence of Ag/ZnO. Dye solution underwent a decolourization and the reaction was monitored spectrophotometrically by measuring the absorbance of dyes at special wavelength. The experimental variables included amount of photocatalyst, concentration of H2O2 and initial concentration of dyes. More than 90% of 100 mgl-1 Reactive Blue 49 decolourization in 30-minute reaction time.
KEYWORDS:Dye Pollutant; Photodegradation; Nanocatalyst; UV-irradiation
Download this article as:| Copy the following to cite this article: Tabatabaee M, Mirrahimi S. A. Photodegradation of Dye Pollutant on Ag/ZnO Nanocatalyst under UV-irradiation. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Tabatabaee M, Mirrahimi S. A. Photodegradation of Dye Pollutant on Ag/ZnO Nanocatalyst under UV-irradiation. Available from: http://www.orientjchem.org/?p=11652 |
Introduction
Textile industries produce large volume of colored dye effluents which are toxic and non-biodegradable. Reactive dyes are commercially, a very important class of textile dyes, whose losses through processing are particularly significant and difficult to treat. It is estimated that about 20-50 percent of the reactive dyes is lost during the dyeing (1). For the treatment of dye-containing wastewater, various biological, physical and chemical methods such as microbial biodegradation, membrane filtration, oxidation, ozonation, adsorption, and ultra filtration have been used (2-5). However, many of these technologies are cost prohibitive, especially when applied for treating large waste streams and some of them merely transfer dyes from the liquid- to the solid-phase, requiring further treatment and causing secondary pollution (6). Thus, there is a need for developing treatment methods that are more effective in elimination dyes from wastewater. Advanced oxidation process (AOPs) is alternative method for the complete degradation many organic pollutants (7-10). When a photocatalyst absorbs radiation whose energy hν>Eg (Eg is the semiconductor band gap energy), an ē from its filled valance band (VB) is promoted to its conduction band (CB) and valance band holes h+ are formed (figure 1). Electron would reduce any available species, including O2, water and hydroxide ion to form hydroxyl radicals. The OH.- radicals are very strong oxidizing agents and can easily attack the organic molecules, Thus leading finally to their complete mineralization.
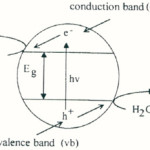 |
Figure 1: The photocatalyst interface under UV- illumination. |
Among the various semiconductors employed as photocatalyst, TiO2 and ZnO are the most preferable material due to their non-toxic, insoluble, stability and high photoactivity properties. Recently semiconductor-based heterostructures have been attracted, due to their potential applications in various fields. Metal/semiconductor is one of the most popular heterostructures and has been extensively studied because of its excellent catalytic activity. Ag/ZnO is one of the heterostructure photocatalyst with high catalytic activity that has attracted much research attention [11]. In this communication, we wish to report synthesis and application of Ag/ZnO nanoparticles as an efficient photocatalyst on degradation of reactive blue 49 (RB49).
Materials and methods
Reactive dye Blue 49 was commercial products and used without further purification. It’s chemical structure is shown in scheme 1. Ag/ZnO was prepared according to litterateurs procedures [12]. A general photocatalytic procedure was carried out as follows: 0.02 g of catalyst Ag/ZnO was suspended in an aqueous RB49 (100 mg/L, pH = 6.8) and appropriate H2O2 solution. The suspension (20 ml) was stirred in the dark for 30 min to obtain establish adsorption–desorption equilibrium between the dye and the surface of the catalyst. The degradation experiment was carried out using Philips HPK 400 W lamp. The temperature was kept at 30+2 ºC. At the specific time, the colloid solutions of Ag/ZnO particles were separated by centrifuged and filtered. Decreasing in the concentration of dye was monitored with a Shimadzu Model 160-A UV-VIS spectrophotometer with a 1-cm quarts cell at λ = 595 nm. All photocalytic experiments were carried out at 6.8 pH. For The degree of photodegradation (X) as a function of time is given by:
X=Co -C/Co
where C0 is the initial concentration of RB49, and C the concentration of RB49 at time t.
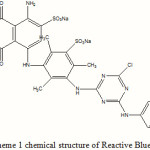 |
Scheme 1: chemical structure of Reactive Blue 49 |
Results and discussion
Figure 2 shows the XRD patterns of the ZnO/Ag composites. In figure 2, three additional peaks at 36.18°, 48.36° and 68.58° compared to pure hexagonal-wurtzite ZnO (JCPDS Card Pattern: 3-888), can be assigned to the face-centered cubic (fcc) structure of Ag crystallite (JCPDS Card Pattern: 1-1167), revealing that fcc structured Ag is produced in the reaction.
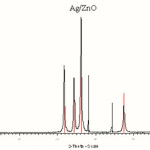 |
Figure 2: XRD patterns of the synthesized Ag/ZnO nanoparticles. |
The changes in absorption spectra of dye during UV/Ag/ZnO process at different time irradiation intervals were shown in figure 3. The decrease of the absorption band of RB49 at λ = 595 nm indicates a rapid degradation of dye. Complete discoloration of dye was observed after 60 minutes in the optimized conditions.
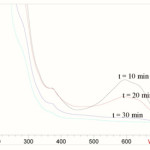 |
Figure 3: UV-Vis spectral changes of RB49, recorded during the dye degradation at different irradiation times, (dye: 100mgL-1, Ag/ZnO: 1 g/L, H2O2:10 mmolL-1). |
Effect of UV irradiation and Ag/ZnO nanoparticles
At first, experiments concerning the decomposition of dye (100 mgL-1) were performed in the presence of Ag/ZnO semiconductor, illumination and H2O2, a blank experiment in the absence of semiconductor and another blank experiment in the absence of hν. Results of these experiments (Fig. 4) show that degradation of dye in the presence of the photocatalyst, irradiation and H2O2 could lead to the disappearance approximately 94% of dye after 30 min. This was contrasted with 11% degradation for the same experiment performed in the absence of Ag/ZnO and 32% in the absence of H2O2. A blank experiment in the absence of illumination demonstrated approximately 13% during of stirring of dyes and photocatalyst in dark and it is due to adsorption–desorption equilibrium between the dye and the surface of the catalyst.
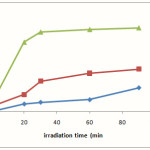 |
Figure 4: plot of the degree of photodegradation (X) vs irradiation time. a: dye (100mgL-1), H2O2 (10 mmolL-1) and UV irradiation. b: dye (100mgL-1), Ag/ZnO 1 g/L and UV irradiation. c: (100mgL-1), Ag/ZnO 1g/L, H2O2 (10 mmolL-1) and UV irradiation. |
Effect of catalyst concentration
The effect of the amount of Ag/ZnO on the photodegradation efficiency was shown in Figure 5. Experiments performed with different concentrations of synthesized Ag/ZnO showed that the photodegradation efficiency increases with an increase in photocatalyst concentration up to 160 ppm, and is then decreased. This observation can be explained in terms of availability of active sites on the catalyst surface and the penetration of UV light into the suspension [13].
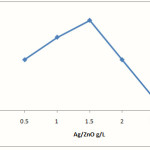 |
Figure 5: photodegradation of RB49 vs different Ag/ZnO concentration (dye (100mgL-1), H2O2 (10 mmolL-1) and irradiation time (30 min). |
Effect of hydrogen peroxide
The rates and efficiencies of photoassisted degradation of organic substrates are significantly improved in the presence of peroxides [14, 15]. The photocatalytic degradation of RB49 has been studied at different hydrogen peroxide concentrations. Results show that the degradation rate of RB49 increased with increasing H2O2 concentration up to 12 mmol/L, but above it, the degradation rate decreased. H2O2 can be converted to hydroxyl radical (Eq. 1). But at high amounts of H2O2, it can act as scavenger of ·OH according to Eq. 2 [16].
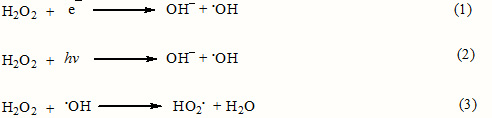
Degradation of dye at various dye concentration
Figure 6 shows the photolysis of various initial concentrations of RB49 in the presence of Ag/ZnO (1 gL-1) and appropriate of H2O2. A little adsorption was observed during of stirring of dye after 30 minutes in dark.
Study of Kinetic modeling
According to many researchers the kinetics of the photocatalytic degradation rate of most organic compounds is described by a pseudo-first kinetic order [13, 14].
As was shown in Fig 6, the plot of ln[dye] versus irradiation time for RB49 is linear and can be assigned that the photodegradation reaction approximately follows the first order kinetics and rate constant (K ) is 0.57 min−1.
Conclusion
In this work the photocatalytic oxidative degradations of a reactive dye (RB49) has been studied. It was observed that, heterogonous photocatalyst (Ag/ZnO) can degrade dye. Approximately 90-95% dyes have been eliminated after 30 minutes in presences of Ag/ZnO as catalyst, H2O2 and optimize conditions
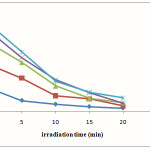 |
Figure 6: Photolysis of various initial concentrations of RB49 in the presence of Ag/ZnO (1 gL-1) and H2O2 (12 mmol/L). |
Acknowledgement
The authors wish to express their gratitude to Islamic Azad University Yazd branch (Grant title: Synthesis of Ag/ZnO nanoparticles and Photocatalytic effect on the Degradation of reactive Dyes) for the support of this work.
References
- Ncibi M. C. Mahjoub B. and Seffen M., Int. J. Environ. Sci. Tech., 4, 433 (2007).
- Forgacs E. Cserhatia T. and Oros G., Environ. Int. 30, 953 (2004).
- Gharbani O., Tabatabaii S. M., Mehrizad A., Int. J. Environ. Sci. Tech., 5, 495 (2008).
- Beydilli M. I., Pavolsathis S.G., Tincher W.C., Water Sci. Tech. 38, 225 (1998).
- Ghasemi F., Tabandeh F., Bambai B. and Sambasiva Rao, K. R. S. Int. J. Environ. Sci. Tech., 7, 457 (2010).
- Igbinosa E.O. and Okoh A. I. Int. J. Environ. Sci. Tech., 6, 175 (2009).
- Kansal S.K., Singh M. and Sud. D. J. Hazard. mater., 141, 581 (2007).
- Giri R. R., Ozaki, H., Taniguchi, S. and Takanami, R. Int. J. Environ. Sci. Tech. 5 17 (2008).
- Hoffman M., Martin S. and Choi W. and Bahnemann D., Chem. Rev. 95, 69 (1995).
- Poulios, I. and Tsachpinis, I. J. Chem. Technol. Biotechnol., 74, 349 (1999).
- Zheng Y., Chen, C., Zhan, Y., Lin, X., Zheng, Q., Wei, K. and Zhu J., J. Phys. Chem. C, 112, 10773 (2008).
- Zhou, G., Deng J., Mater. Sci. in Semicond. Process., 10, 90, (2007).
- Daneshvar, N., Salari, D. and Khataee, A.R., J. Photochem. Photobiol.A: Chemistry 162, 317 (2004).
- Daneshvar, N., Salary, D. and Behnasuady, M.A., Iran J. Chem. Chem. Eng. 21, 55 (2002).
- Fox, M.A. and Dulag, M.T., Chem. Rev. 93, 341 (1993).
- Daneshvar, N., Salari, D. and Khataee, A.R., J. Photochem. Photobiol. A 157, 111 (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.









