Preparation and Analytical Properties of 4-Hydroxybenzaldehyde, Biuret and Formaldehyde Terpolymer Resin
Bunian A. Shareef1*, Ibrahim F. Waheed2, Kariem K. Jalaot3
1Department of Ninava, Ministry of Science & Technology, Iraq. 2Department of chemistry, Tikrit University, Iraq. 3Environmental directorate, Ministry of Science & Technology , Iraq.
DOI : http://dx.doi.org/10.13005/ojc/290414
Article Received on :
Article Accepted on :
Article Published : 08 Dec 2013
The chelating resin was synthesized by polycondensation reaction of 4-hydroxy benzaldehyde, formaldehyde and biuret under alkaline condition. The prepared resin was characterized by IR spectroscopy and 1HNMR spectroscopy. The resin sample was cured isothermally at 1200C.The DSC scans was measured for the prepared resin. The analytical evaluation of the prepared resin toward the studied ions (Hg2+, Ni2+ and Pb2+) using the batch method. It was employed to study selectivity of metal ion uptake in a different pH and different treatment time.
KEYWORDS:synthesis;terpolymer;chelating resins;removal of heavy metals
Download this article as:| Copy the following to cite this article: Shareef B. A, Waheed I. F, Jalaot K. J. Preparation and Analytical Properties of 4-Hydroxybenzaldehyde, Biuret and Formaldehyde Terpolymer Resin. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Shareef B. A, Waheed I. F, Jalaot K. J. Preparation and Analytical Properties of 4-Hydroxybenzaldehyde, Biuret and Formaldehyde Terpolymer Resin. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1136 |
Introduction
Removal and separation of metal ions in aqueous solution play an important role for the analysis of wastewaters, industrial and geological samples as well as for environmental remediation[1]. Chelating resins have been widely utilized for the removal of the undesired metal ions from this aqueous solution[4-5]. Toxic heavy metal ions are well know their serious harm to human health. If the toxic metal ions can be recovered, the energy and material requirements of the wastewater treatment process can be simpler. The use of chelating resins for separation and removal ions is the method of choice due to its highly separation efficiency, good reproducibility of retention parameters and simplicity of operation[6]. Therefore, the development of high performance chelating resins for removing heavy metal ions from aqueous solution is considered not only as research priority in the environmental field but also as an area of interest inorganic catalyst, recovery of valuable trace metal ions[7]. Two prepolymer types are obtained depending on pH, novolacs in an acidic pH region whereas resols by alkaline reaction. Resol resins are synthesized with a molar excess of formaldehyde. These are mono- or polynuclear hydroxymethylphenols which are stable at room temperatures, but are transformed into three dimensional, cross linked, insoluble and infusible polymer by the application of heat[8-9]. In the present paper we describe the synthesis and characterization of chelating 4-hydroxy benzaldehyde –formaldehyde–biuret. The resin has been studied for sorption of Hg(II), Ni(II), Pb(II) from aqueous solutions. For this purpose, various factors affecting the chelation efficiency such as pH and treatment time. The thermal properties have been studied by using DSC analysis.
Experimental
Materials
The starting materials used were obtained from different sources and companies like 4-hydroxybenzaldehyde and biuret (Fluka co., Germany), formaldehyde, absolute ethanol and nitric acid (BDH co., England), sodium hydroxide (Merck co., Germany). The stock standard solutions of Pb(II), Ni(II), Hg(II) were prepared by dissolving of appropriate amount of Pb(II),Ni(II),Hg(II) nitrates in deionized water, acidified with small amount of corresponding acid.
Terpolymerization
The terpolymer resin was prepared by condensation polymerization of 4-hydroxybenzaldehyde (0.1mol) and biuret (0.1mol) with formaldehyde (0.2mol), sodium hydroxide medium at 1200C in an isomental heater for 3hs. The solid product obtained was immediately removed from the flask, washed with cold water, dried and powdered. The powder was repeatedly washed with hot water to remove excess of 4-hydroxybenzaldehyde- formaldehyde terpolymer, which might be present with the 4-HBFB terpolymer.
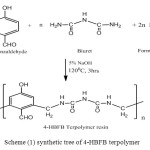 |
Scheme (1) synthetic tree of 4-HBFB terpolymer Click here to View figure |
Spectral analyses
The FTIR spectrum of the prepared terpolymer had been scanned in KBr pellets on a shimadzu 8400 spectrophotometer, (at science collage, Tikrit university).1HNMR spectrum of the terpolymer was carried out in a Bruker ultra shield 300MHz spectrophotometer using DMSO-d6 as a solvent, at al-albiat university, Jordan.
Analytical study
The chelation efficiency properties of 4-HBFB terpolymer resin was evaluated by batch method for specific metal ions such as Hg2+, Ni2+and Pb2+ in form of their aqueous metal nitrate solutions. The procedure adopted on removes of heavy metal ions by shaking of the prepared terpolymer with 5ml solution having 100µg/ml metal ion concentration. In each case pH was adjusted to a specific value (1,2 and 3) for Hg2+ ions and (2,4 and 6) for Ni2+ and Pb2+ ions. For different treatment time intervals ranging from 1h to 24hrs. Finally, the solutions were filtered through filter paper and the residual metal ions concentration in the filtrate was measured by using atomic absorption spectrophotometer (A Varian AA-10).
Results and Discussion
The resin sample was light pink in color, insoluble in commonly used organic solvents, not soluble in H2SO4 and hardly soluble in DMSO. The melting point of this resin was determined by electrically heated melting point apparatus and is found to be in the range of 162-1650C.
Spectral studies
The IR spectrum of the 4-HBFB terpolymer resin are presented in Fig1. The band which appeared in the region 3392-3365cm-1 may be assigned to the stretching vibration of the phenolic hydroxyl group exhibiting intermolecular hydrogen bonding. The presence of weak peak at 2887 cm-1 describe the -NH- in biuret moiety may be a scribe in the terpolymer chain. The presence of methyl and methylene vibrations at 2929-2856cm-1 gives sharp and weak peaks. The sharp band displayed at 1670cm-1 may be due to the stretching vibration of carbonyl group of both ketonic as well as biuret moiety. The sharp and weak bonds obtained at 1388, 1230 and 806cm-1 suggested the presence of methylene bridge in the terpolymer chain. 1,3,4,5-tetra substitution of aromatic ring is recognized from the bands appearing at 1018 and 758cm-1 are assumed C-H binding vibration.
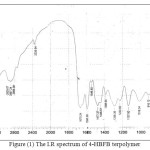 |
Figure (1) The I.R spectrum of 4-HBFB terpolymer Click here to View figure |
1H-NMR spectra of 4-HBFB terpolymer are shown in Fig.2. 1H-NMR spectra of 4-HBFB terpolymer show weak multiplicity signals (unsymmetrical pattern) in the region (dH=7.0-7.5 ppm) which may be due to the aromatic protons. The weak multiply signals appearing at (dH=5.0-5.7ppm) may due to the amido-CH2-NH-CO linkage. A signal peak appeared in the region (dH=2.5 ppm) may be due to protons of methylene bridges Ar-CH2-N of polymer chain. A weak signal which appeared in the region of (dH=9.5-9.6ppm) may be due to the Ar-CHO group. The signal in the range of (dH=3.4-3.9ppm) is attributed to phenolic proton.
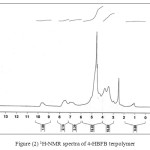 |
Figure (2) 1H-NMR spectra of 4-HBFB terpolymer Click here to View figure |
Thermal analysis
The curing study of the prepared resin was investigated by differential scanning calorimetry technique using DuPont 9100 DSC type. The DSC scans was analyzed several curing parameters were evaluated the data obtained are shown in fig.3. The sample was heated at rate of 100C/min. from 500C to 3000C. Heat of melting gives idea about percent crystallinity of polymer, the melt of amorphous polymer has been range of melting points result from different crystalline regions in polymer structure. Is expected to be the first peak at 760C showing some physical changes in polymer due to refractive of hydrogen bonding between the polymer chains either the second peak at 2370C is expected to be happen some chemical changes such as losses of water molecules from the crystalline polymer structure.
 |
Figure (3) differential scanning calorimetry (DSC) of 4-HBFB terpolymer Click here to View figure |
Chelating efficiency
The loading capacity is presented by amount of metal ion chelated or adsorbed by the terpolymer in g ion/g resin or mg ion/g resin[10].(126 thesis)
 |
Figure 4 – 9 Chelating efficiency Click here to View figure |
The effect of pH on loading capacities
Due to the protonation and deprotonation of terpolymer groups, its chelation behavior for metal ions influenced by the pH values as shown in fig.4, fig.6 and fig.8. From this figs. at strongly acidic pH the terpolymer has lower loading capacity(L.C.) than that at higher one. This can be explained by the fact that at lower pH values, most of terpolymer groups are protonated. Then cationic repulsion can occur between metal ion species and protonated groups in terpolymer. The maximum L.C. at pH 6 for Pb2+ and Ni2+, the maximum L.C. for Hg2+ at pH 3. The lower L.C. of Pb2+ than Hg2+ and Ni2+ may be explained due to greater hydrated ion radius of Pb2+ than that of Hg2+ and Ni2+. This will result in lower electrostatic interaction between Pb2+ and the coordinating groups and hence lower complex stability and therefore exhibits lower capacity.
The effect of treatment time on loading capacities
Fig.5, fig.7 and fig.9 shows the effect of treatment time on L.C. of metal ion. L.C. increases by increasing the treatment time during the 6h, then level off by increasing treatment time to 24h. We notice the decrease of L.C. for prepared resin toward Hg2+ and Ni2+ at lower pH although increasing the treatment time this may be due to at low pH of solution increase of H3O+ ions concentration and intensifies the competition between H3O+ ions and heavy metal ions for complexation sites.
Regeneration of the loaded resin
Resins loaded with highest amount of ions were chosen for the regeneration study and metal ion recovery. Hydrochloric acid (2M) was used as an fluent for the studied ions. The amount of the recovered ions depends mostly on the nature of the ion itself. The percentage ion recovery of the studied ions was in the following order: Ni2+ ˃Pb2+ ˃Hg2+.
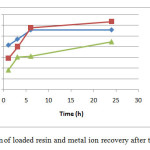 |
Figure (10) Regeneration of loaded resin and metal ion recovery after treatment with 2M HCl Click here to View figure |
Conclusion
- A resin 4-HBFB based on condensation reaction of 4-hydroxybenzaldehyde and biuret with formaldehyde in presence of sodium hydroxide catalyst has been prepared.
- The resin showed a higher selectivity toward Hg2+ ions compared to Ni2+ and Pb2+.
- Due to the presence of O and N atoms may be plays the key role in binding the metal ions and helps in chelating.
- 4-HBFB terpolymer has application as an chelating resin in waste water treatment and metal ions recovery.
Acknowledgment
This work was done by Iraqi ministry of science and technology/provinces affairs directorate/Ninava branch and in collaboration with the Faculty of Science/Tikrit University. One of authors thanks to Dr. Azhar A. Mohammad for support and encouragement.
References
- Shah, B.A. , Shah, A.V. , Shah, P.M. (2009). “Analytical and morphology studies of phthalic of acid-formaldehyde resorcinol as chelating resin”. Malaysian Polymer Journal, Vol. 4, No. 2, p 1-12.
- Bisset W, Jacobs H, Koshti N, Stark P, Gopalan A.( 2003). “synthesis and metal ion comlexation properties of a novel polyethyleneimine N-methylhydroxamic acid water soluble polymer. React Funct Polym. 55(2):109-119.
- D. Dikshit., Orient. J. Chem., 29(1), 305-307, (2013).
- Geckeler KE, Volchek K. (1996). “Removal of hazardous substances from water using ultrafiltration in conjunction with soluble polymers. Environ Sci Technol. 30(3):725-734.
- Smith BF, Gibson RR, jarvinen GD, Robison TW, Shroeder NC, Stalnaker ND. (1998). “Preconcentration of low levels of americium and plutonium from waste waters by synthetic water soluble metal-binding polymers with ultra filtration”. J radioanal Nucl Chem. 234(1-2):225-229.
- Yirikoglu, H. and Gulfen, M. (2008). “separation and recovery of silver (I) ions from base metal ions by melamine-formaldehyde-thiourea (MFT) chelating resin”. Separation science and technology. 4:376-388.
- Cheng-Chien Wang,1 Chun-Chih Wang2. (2005). “synthesis and characterization of chelating resins with amino moieties and application on removal of copper(II) from EDTA complexes”. Inc. J Appl Polym Sci. 97: 2457-2468.
- H. Hosseinzadeh., Orient J. Chem., 29(1), 161-166, (2013).
- Ida Poljanšek and Matjaž Krajnc. (2005). “characterization of phenol-formaldehyde prepolymer resins by in line FT-IR spectroscopy”. Acta Chim. Slov. 52, 238-244.
- M.Kaneko, E. Tsuchida, (1981). “Formation, characterization, and catalytic activities of polymer–metal complexes” J. Polyme. Sci. Macromolecular reviews. 16, 397.

This work is licensed under a Creative Commons Attribution 4.0 International License.









