Compare Removal of Reactive Red195 and Blue19 by Nano TiO2-ZnO
Z. Pournuroz1, M. Khosravi1*, M. Giahi2 and M. Sohrabi1
1Department of Chemistry, Tehran north Branch, Islamic Azad University, Tehran, Iran.
2Department of Chemistry, Lahijan Branch, Islamic Azad University, Lahijan, Iran.
Corresponding Author:E-mail: khosravim059@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310351
Article Received on :
Article Accepted on :
Article Published : 15 Sep 2015
In this study, TiO2-ZnO particles were synthesized by the sol-gel method. XRD, SEM, EDX and UV-vis techniques were used to characterize optimize doped TiO2-ZnO sample Aim of this research is investigation of possibility of mixture TiO2-ZnO in degradation reactive dye. In addition, Influence of effective parameters on photodegradation reaction such as TiO2-ZnO concentration and initial concentration of dyes on the removal were investigated. Results showed that removal efficiency of dyes by increasing of TiO2-ZnO concentration was increased. In addition, the best dye removal for both dyes were obtained 11mg.L-1.
KEYWORDS:photocatalytic degradation; Reactive Dye; nano particle TiO2-ZnO
Download this article as:| Copy the following to cite this article: Pournuroz Z, Khosravi M, Giahi M, Sohrabi M. Compare Removal of Reactive Red195 and Blue19 by Nano TiO2-ZnO. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Pournuroz Z, Khosravi M, Giahi M, Sohrabi M. Compare Removal of Reactive Red195 and Blue19 by Nano TiO2-ZnO. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10920 |
Introduction
Azo dyes having aromatic structure, are resistant to biodegradation. They can also reduce amount of oxygen the water, Whereby Increases the activity of anaerobic bacteria in the water[1]. However, Removal dye of the wastewater is more important than the removal of non-colored organic matter, Because The dye concentration in receptive waters, wastewater may be less than 1mg.L-1.But the dye, visible even in such low concentrations and Their environmental effects will be followed[2]. According issues raised Existing waste water treatment Contain Amount Organic dyes With new methods, Effective and Industrial are imperative And required. One of the best ways to remove pollutants using advanced oxidation (AOPs) such as photocatalysts is TiO2[3]. Titanium dioxide is a compound that has high photocatalytic activity. And a lot of research for the study and application of this important characteristic of titanium dioxide has been done[4-7]. Also titanium dioxide Due Chemical stability, Non-toxicity And Low Cost A lot of considered. However, the application of photocatalytic titanium dioxide has been limited for two reasons[8].
- The band gap of titanium dioxide is in the ultraviolet region. While Only 3% of The spectrum sunlight is Area Ultraviolet[9].
- 2. Pairing fast electron – hole in photocatalytic activity of titanium dioxide causing the loss it.
Therefore,In recent years, various methods for improving energy band gap and increase the optical activity of titanium dioxide that has been done, including reduce the particle size, increased specific surface area, a pair of titanium dioxide with other semiconductor refine of titanium dioxide with metals and non-metals include titanium dioxide and increased activity[10-16].
In this research, the effect of doped Tio2 with ZnO for degradation two important dye(for dying cotton, rayon and wool) in the textile industry was studied.
Experimental
Materials
The Reactive Blue 19 was purchased from the Dystar (Germany) and The Reactive Red 195 was purchased from the SUN COLOUR (Korean) and used without further purification. Solutions were prepared with the dye using distilled deionized water (Table1).
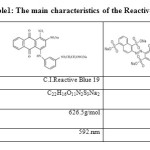 |
Table 1: The main characteristics of the Reactive dye |
Titanume (IV) isoporopoxid (TTIP) Zinc nitrate hexahydrate (Zn(NO3)2·6H2O), Fe(NO3)3·9H2O, glacial acetic acid, etanol, “Degussa-P25” with size 30 nm were from Merk chemical Company and Zno with particle size 20nm were from nano more company., were from Merk chemical Company. The pH of the solution was adjusted with HCl or NaOH.
Preparation of nanocoposites
The TiO2/ZnO were prepared through the sol–gel process [17-20]. Precursor A: 12.83 ml (C4H9O)4Ti and 14.60 ml C2H5OH were mixed together and stirred for 1.0 h at room temperatures. Precursor B: 1.35 ml H2O, 7.30 ml C2H5OH, 2.20 ml CH3COOH and a proper amount of zinc nitrate (with TiO2:ZnO=10:3) were mixed together with Fast stirring. Meanwhile, a certain quantity of hydrochloric acid (HCl) solution was also added for controlling the solution pH value between 2.0 and 3.0. Here, acid was used as an inhibitor to reduce quick hydrolysis of TTIP. Afterwards, the precursor B was dropped into the precursor A with vigorous stirring. The resultant mixture was further stirred for 1 h and the yellow transparent sol was gained. The obtained gel was dried at 60ºC for 10 h in a vacuum oven and ground to obtain powder. The heat-treatment of 400ºC for 2.5 h in the air was applied to the sample.
Photocatalytic activity measurement
The photodegradation studies were carried out in a batch reactor system. The setup consists of a UV chamber made of MDF having dimensions (68 × 50 × 43 cm) and two lamps TUV PL-L 18 W (Philips), fitted on the top of the chamber. An exhaust fan is fitted on the side wall of the chamber to maintain a constant temperature. The reactor used is a cylindrical quartz flask, which has a diameter of 2.5cm and is 15 cm in height. To characterize the photocatalytic activity of the nano composite under UV-C irradiation, measurements on photodegradation of reactive dyes in the model wastewater were carried out at room temperature.
For each experiment, 11mg nano-composite was put in a cylindrical flask containing 25 ml, water solution of Reactive dyes whit concentration 20 mgL-1. The solution was magnetically stirred for 30min in dark to establish an adsorption/desorption equilibrium of Reactive dyes on the surfaces of the catalyst, then the light was turned on to initiate the photocatalytic reaction. After irradiation for 45 minute, the suspension was extracted at regular time (15 min) and centrifuged at the rate of 4000 rpm for 5 min to remove the catalyst. The concentrations of Reactive red 195 and blue19 before and after photocatalytic degradation were calibrated by the intensity of its characteristic absorption peak located at 542 and 592 nm measured with UV–vis spectrometer Respectively (Jenway 6405).
The degree of photodegradation (X) as a function of time is given by:
%Degradation X = (1-C/C0)´100 (1)
where C0 is the concentration of Reactive dyes before illumination and C is the concentration after irradiation time.
Results and Discussion
Characterization of TiO2/ZnO
Figure 1 shows X-Ray Diffraction (XRD) patterns of the TiO2-ZnO nano particle fabricated. peaks observed at 2q=25.30°, 36.21°, 47.59°, 53.69° (Fig. 1). using the XRD peak at 2q=25 (anatase), 2q= 36 (zincite).
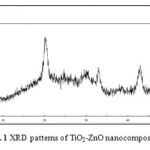 |
Figure 1: XRD patterns of TiO2-ZnO nanocomposite |
we use the Energy dispersive X-ray (EDX) spectrum. The element of Titanium, zinc and oxygen were determined as well in (Fig.2).
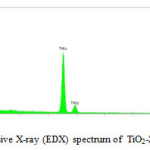 |
Figure 2: Energy dispersive X-ray (EDX) spectrum of TiO2-ZnO nanocomposite. |
Average particle size was determined using Debye-Scherrer formula with Full-width at Half Maximum (FWHM). The sizes were found to be 23.45 nm. Morphology of TiO2-ZnO nano particles was evaluated by Scanning Electron Microscopy (SEM )The SEM images of nano TiO2-ZnO prepared is shown in Figure. 3
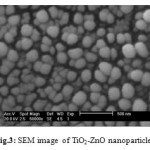 |
Figure 3: SEM image of TiO2-ZnO nanoparticles |
At first Nano-photocatalysts TiO2/ZnO synthesized by sol-gel method, result shown that degradation of reactive dye by nano (TiO2-ZnO) more than TiO2 and ZnO alone. (Fig4)
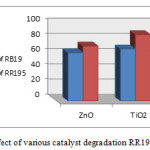 |
Figure 4: The effect of various catalyst degradation RR195 and RB19 |
The effects of nano photocatalyst in the degradation of dye
Studies show that by increasing the amount of nanophotocatalysts to 11 mg.L-1 maximum destruction have for both dyes.Further increasing the amount of nanophotocatalysts appears that the nanoparticles (the suspended particles) create error in the assessment of their destruction.
Figure 5 shows a typical time-dependent UV spectrum of Reactive dyes solution during photoirradiation. Reduce the degradation of RR195 indicates the presence of azo bond (-N=N-) at the RR195 caused the structure more susceptible than RB19 is attacked by hydroxyl radicals.
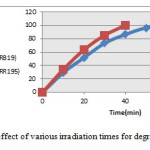 |
Figure 5: effect of various irradiation times for degradation |
Effect of Concentration of Reactive Dye
Effect of initial dyes concentration on degradation was examined in the range of 10-50 mg.L-1 at 11mg.L-1 catalyst loading under UV irradiation shown in Figure 6.
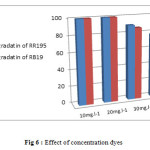 |
Figure 6: Effect of concentration dyes |
It was found that the rate of degradation of the dyes decreased at higher concentrations. With increasing the amounts of RR195 and RB19, more of the dyes molecules will be adsorbed on the surface of the photocatalyst and the active sites of the photocatalysts will be reduced. so, with increasing occupied space of photocatalyst surface, the generation of hydroxyl radicals will be decreased. Also, increasing concentration of dyes can be lead to decreasing the number of photons that is arrived to the surface of catalysts. As more light is absorbed by molecules of dyes, the excitation of photocatalyst particles by photons will be reduced. Therefore, degradation efficiency would be diminished.
the kinetics of disappearance of RB19 and RR195 for an initial concentration of 20 mg.L-1 under optimized conditions show in (fig. 7). The results showed that the photocatalytic degradation of the dyes in aqueous solution TiO2-ZnO can be described by the first order kinetic model as ln (C0/C) = kt, where C0 is the initial concentration and C is the concentration at any time [21]. The semi logarithmic plots of the concentration data gave rise to a straight line. The correlation constant for the fitted line and rate constant were calculated RB19 and RR195 as R2 =0.998, 0.997 and 0.068 ,0.094 min-1 respectively. According to results Rate constant of RR195 was more RB19.
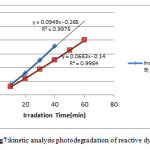 |
Figure 7: kinetic analysis photodegradation of reactive dyes |
Reference
- Tang, W.; An, H. Chemosphere,1995, 31, 4157-4170.
- Grzechulska, J., Morawski, A.W. Appl. Catal. B, 2002, 36, 45-51.
- Hernandez, F.; Revera, A.; Romero, O.; and et al. Oriental Journal of Chemistry, 2014, 30,4, 1545-1551
- Rezig, W.; Hadjel,M. Oriental Journal of Chemistry, 2014, 30,4,993-1007
- Chong, M. N.; Jin, B.; Chow, C. W. K.; Saint, C. Water Res.2010, 44, 2997-3027.
- Zare-Hossein-abadi, D.; Langroudi, A. E.; Rahimi, A. J. Color Sci. Technol,2009, 3, 121-129
- Paolaa, A. D.; Cufaloa,G.; Addamoa, M.; and et al. Colloids Surf. A. 2008,317, 366–376.
- Konstantinou, I, K.; Albanis,T,A. Appl. Catal. B.2004, 49, 1-14.
- Yamasaki, R. S. J. Paint Technology.1971, 43, 75-83.
- Hukovi,M. M.; Ceri, M.C. Surf. Sci.1985, 24, 273-283.
- Gervais,F.; Baumard, J, F. Solid State Commun. 1977, 21, 861-865.
- Millot,F.; Picard, C. Solid State Ionics. 1988, 28-30, Part 2, 1344-1348.
- Marucco, J, F.; Gautron, J.; Lemasson, P. Solid State Commun.1981, 37, 2-3.
- Sonawane, R. S.; Dongare, M. K. J. Mol. Catal. A: Chem.2006, 243, 68-76.
- Zhu,Y.; Wei,,W.; Dai,Y.; Huang,B. Appl. Surf. Sci. 2012, 258, 4806-4812.
- Kokila, P.; Senthilkumar,,V; Nazeer,K.P. Arch. Phys. Res.2011, 2, 246-253.
- Joanna ,R.; Anna ,I.; Gerard, S.; Adriana ,Z. Physicochem. Probl. Miner Process, 2012, 48, 201.
- Jingqun ,Go.; Xiaoyu, L.; Jun, W.; Baoxin. Desalination. 2011, 268, 68.
- Wang, J.; Li ,R.H.; Zhang, Z.H.; Sun, W. Appl.Catal. A. 2007, 334, 227.
- Morales,B.A.; Novaro,O.; Sanchez, E.;Gomez ,R.J. Mater. Res. 1995, 10, 2788.
- Sayılkan, F.; Asilturk, M.; Tatar, P.; Kiraz, N.; ener,S.; Arpac, E.; Sayılkan, H. Mater. Res. Bull. 2008, 43, 127.

This work is licensed under a Creative Commons Attribution 4.0 International License.









