Synthesis, Characterization, Anti-Oxidant and Anti Inflammatory Activity Evaluation of Chalcones & Pyrazoline Derivatives
L. Siva Sanker Reddy *,T. Rajkumar, G. Lakshmi Mrudula and Y. Siva Rami Reddy
Department of Pharmaceutical Chemistry, Creative Educational Society’s College of Pharmacy, NH-7, Chinnatekur, Kurnool. Andhra Pradesh. 518218.
Correspondence Author Email: Shiva_s_rl@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/31.Special-Issue1.23
Article Received on :
Article Accepted on :
Article Published : 07 Sep 2015
Pyrazolines display a broad spectrum of potential pharmacological activities. Hence pyrazolines are used extensively as useful synthons in organic synthesis. 4-Amino acetophenone was diazotized then followed by coupling with morpholine as a means of protection of amine group in 4-Amino acetophenone. The obtained product is then made to react with different aldehydes in the presence of 40% KOH solution as catalyst to yield chalconederivatives. These are then subjected for cyclization by treating with hydrazine hydrate. The structures were proposed based on 1H NMR and IR spectral data. All the compounds are screened for Anti-oxidant and anti Inflammatory activity.
KEYWORDS:Chalcones; 4-Amino acetophenone; Pyrazolines; Anti-oxidant; Anti- Inflammatory
Download this article as:| Copy the following to cite this article: Reddy L. S. S, Rajkumar T, Mrudula G. L, Reddy Y. S. R. Synthesis, Characterization, Anti-Oxidant and Anti Inflammatory Activity Evaluation of Chalcones & Pyrazoline Derivatives. Orient J Chem 2015;31(Special Issue1). |
| Copy the following to cite this URL: Reddy L. S. S, Rajkumar T, Mrudula G. L, Reddy Y. S. R. Synthesis, Characterization, Anti-Oxidant and Anti Inflammatory Activity Evaluation of Chalcones & Pyrazoline Derivatives. Orient J Chem 2015;31(Special Issue1). Available from: http://www.orientjchem.org/?p=10640 |
Introduction
In Medicinal chemistry, derivatives of 4-Amino Acetophenone found to have diverse therapeutic applications. Many 4-Amino Acetophenone derivatives have been developed as chemo therapeutic agents and are widely used. 4-Amino Acetophenone moiety carrying compounds exhibit various activities like anti-bacterial, anti-fungal, anticancer, anti-convulsion, anti-inflammatory, anti-oxidantetc1,2,3,4.
Chalcone is an aromatic ketone and an enone that forms the central core for a variety of important biological compounds, which are known collectively as chalcones or chalconoids. These are coloured compounds because of the presence of the chromophore -CO-CH=CH-. Chalcones bears a very good synthon so that variety of novel heterocycles with good pharmaceutical profile can be designed. Chalcones can be prepared by Claisen-Schmidt condensation between an aromatic aldehyde and an aromatic ketone in the presence of sodium hydroxide as a catalyst.
Chalcones are popular intermediates for synthesizing various heterocyclic compounds. The compounds with the backbone of chalcones have been reported to possess various biological activities such as anti-microbial, anti-inflammatory of chemical mediators release, inhibition of leukotriene B4, inhibition of tyrosinases an inhibition of aldose reductase activities. The presence of a reactive α,β-unsaturated keto function in chalcones is found to be responsible for their biological activities5.
Heterocyclic compounds are well known for their wide range of biological applications out of which pyrazolines occupy unique position due to dominant applications. Pyrazolines are well known and important nitrogen-containing five-membered heterocyclic compounds. Several pyrazoline derivatives have been found to possess considerable biological activities, which stimulated research activity in this field. Considerable attention has been focused on Pyrazolines and substituted Pyrazolines due to their interesting biological activities. They have found to possess anti-fungal, anti-depressant, anti-convulsant, anti-inflammatory, anti-bacterial, anti-cancer, antioxidant, anti-pyretic, anti-neoplastic activities, anti-viral, anti-amoebic, anti-cholinergic, antidiabetic, anti-HIV, antimalarial, anaxiolytic, antiparasitic, anti-allergic, anti-microbial, anti-tuberculosis, tyrosinase inhibitor, hypoglycemic, hypotensive, immunosuppressive, anti-tumor properties6,7. Pyrazoline is dihydropyrazole which is a five membered heterocyclic compound containing two nitrogen atoms in adjacent position having only one endocyclic double bond. Among all the pyrazoline derivatives 2-pyrazoline has gained the most importance because of its diverse biological activities. 2-Pyrazolines display a broad spectrum of potential pharmacological activities and are present in a number of pharmacologically active molecules such as phenazone/ amidopyrene/ methampyrone (analgesic and antipyretic), azolid/ tandearil (anti-inflammatory), indoxacarb (insecticidal), anturane (uricosuric), etc. In addition, pyrazolines have played a crucial part in the development of theory in hetero cyclic chemistry and also used extensively in organic synthesis.
Materials and Methods
The Identification and characterization of synthesized compounds were carried out by the following procedure to ascertain that all the prepared compounds were of different chemical nature than the respective parent compounds. This involved the determination of the melting point, solubility character’s, and their behaviour in Thin Layer Chromatography (TLC) studies as compared to that of their parent compounds and Nuclear Magnetic Resonance (1H NMR) data, Infra-red spectroscopy. The melting point was determined for the synthesized compounds were taken in open capillary tubes by using Arson digital melting point apparatus which were uncorrected.
The TLC was done on precoated aluminium plates of silica gel 60 F254 obtained from MERCK and visualization of spots was done by using UV TLC visualization chamber.IR Spectra recorded on BRUKER,1H NMR spectra of the compounds recorded on BRUKER-AMX 400 MHz, UV Visible spectrophotometer of Lab India was used for screening the Synthesized compounds for antioxidant activity.
Methodology
Step1: Diazotization and coupling of amine Group in 4-Amino Acetophenone with morpholine:
0.01 mol of 4-Amino acetophenone is dissolved in a mixture of 3ml of Conc. HCl and 6ml water. Then dissolve 1.35gm of sodium nitrite in 6ml of water . Both the above solutions are cooled to 0-5oC by keeping in an ice bath and also by the addition of few pieces of ice to the 4-Amino acetophenone solution. Now sodium nitrite solution is added drop wise to the mixture of 4-Amino acetophenone in Conc. HCl and water with continuous stirring in order to avoid rise in temperature during the reaction. After the completion of sodium nitrite addition, morpholine is added to the reaction mixture with continuous stirring at the same temperature i.e., 0-5oC until a yellow coloured precipitate is obtained. This is then filtered and washed with ice cold water and dried.
Step 2: Preparation of Chalcones
0.005 mol of diazotized 4-Amino Acetophenone coupled with morpholine and equimolar amount of aldehydes in a round bottomed flask containing 50ml of ethanol, and then 1ml of 40% KOH is added at the room temperature with continuous stirring on magnetic stirrer for 2hrs. The reaction mixture is monitored by TLC during the reaction. After the completion of the reaction the reaction mixture is poured into crushed ice with a little amount of dilute HCl in order to neutralize potassium hydroxide. Then the solid is filtered and washed with cold water, dried and recrystallized from ethanol.
Step 3: Preparation of Pyrazolines
0.003mol of chalcones and 0.024mol (1.2ml) of hydrazine hydrate in a round bottomed flask containing 30ml ethanol as a solvent is refluxed for 2hrs at 60oC by monitoring with TLC, After the completion, the reaction mixture is poured into crushed ice and the solid is filtered and dried.
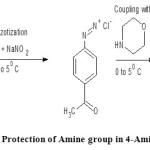 |
Figure 1: Protection of Amine group in 4-Amino Acetophenone Click here to View figure |
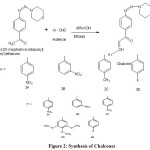 |
Figure 2: Synthesis of Chalcones Click here to View figure |
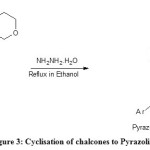 |
Figure 3: Cyclisation of chalcones to Pyrazolines Click here to View figure |
Antioxidant Activity
DPPH Method :(1, 1-diphenyl-2-picrylhydrazyl8
To 3 ml of various concentrations of test/ standard solution, 1 ml solution of DPPH 0.1 mM(0.39 mg in 10 ml methanol) was added. Simultaneously blank samples were prepared for each concentration without addition of 0.1mM of DPPH solution and equal amount of methanol was added to each blank sample. 3 ml of methanol and 1 ml of 0.1mM DPPH was added and used as control. Ascorbic acid was used as standard for comparison. After incubation for 20 minutes in dark, absorbance was recorded at 517 nm. % scavenging was calculated using the formula %Scavenged = [(A-A1)/A] x 100
Where A=Absorbance of the control ; A1=Absorbance of the test or standard
Hydrogen Peroxide Method9
The ability of the synthesized compounds to scavenge H2O2 was determined by the following procedure. A solution of H2O2 (40mM) was prepared in phosphate buffer (pH 7.4). The concentration of H2O2 was determined by absorption at 230 nm using a UV Visible spectrophotometer. Test solutions were added to a H2O2solution (0.6 ml 40mM). The absorbance of H2O2 at 230 nm was determined after 10 minutes against blank solution containing phosphate buffer and test compound without H2O2. Control solution was prepared by taking a solution of H2O2 in phosphate buffer (pH 7.4) and its absorbance was measured. The percentage of H2O2 scavenging by the test and the standard was calculated using the following formula. %Scavenged = [(A-A1)/A] x 100.
Where A=absorbance of the control, A1= absorbance of the test /standard
Invitro Anti-Inflammatory Activity
Inhibition of Egg Albumin Denaturation Method10
To 2ml of various concentrations of test or standard solutions 2.8ml of normal saline (PH=7.4) and 0.2ml of 1% egg albumin solution was added. Simultaneously blank samples were prepared for each concentration without addition of 1% egg albumin solution and equal volume of normal saline (PH=7.4) was added to each blank sample. To 4.8ml of normal saline (PH=7.4), 0.2ml of 1% egg albumin solution was added and used as control. The test/standard samples were incubated for 15min at 70oC. Then the tubes were cooled under running tap water and then absorbance was recorded at 660nm. % inhibition of denaturation of egg albumin was calculated using the following formula.
% Inhibition = [(A-A1)/A] x 100
Where A=absorbance of the control. A1= absorbance of the test /standard.
Heat Induced Haemolytic Method11
To 1ml of various concentrations of test or standard solutions, 1ml of 1% RBC’s suspension was added. Simultaneously blank samples were prepared for each concentration without addition of 1% RBC’s suspension and equal amount of normal saline was added to each blank sample. Equal amount of 1% RBC’s suspension and normal saline was added and used as control. All these samples were taken into centrifuge tubes and incubated in water bath at 56oC for 30 min. The tubes were cooled under running tap water and then centrifuged at 2500 rpm for 15 min and absorbance of supernatant was taken at 560 nm. % inhibition was calculated using formula
% Inhibition = [(A-A1)/A] x 100
Where A=absorbance of the control.
A1= absorbance of the test /standard.
Calculation of IC50 values12
IC50 was calculated using Graphpad prism software. In order to calculate IC50 initially XY data table was created. Then the logarithm of the concentration of the inhibitor was entered into X and response was entered into Y. From the data table click Analyze, choose nonlinear regression, then choose the panel of equations “Dose response curves-Inhibition” and then choose the equation “log (Inhibitor) v/s normalized response-variable slope”. Then we will get IC50 values for the given data.
Results and Discussions
Table 1: Physico-chemical characterization data of synthesized compounds
| S.No. |
Compound Code |
Molecular formula |
Mol.wt |
%Yield |
M.P |
Rf Value |
Colour |
|
1 |
2A |
C19H18N4O4 |
366.37 |
87% |
216-218 °C |
0.47* |
Green |
|
2 |
2B |
C19H18N4O4 |
366.37 |
85% |
136-139°C |
0.7* |
Green |
|
3 |
2C |
C20H21N3O2 |
335.39 |
70% |
137-140°C |
0.57* |
Orange |
|
4 |
2D |
C22H25N3O5 |
411.45 |
86% |
126-129°C |
0.52* |
Green |
|
5 |
2E |
C19H18F N3O2 |
339.36 |
88.20% |
102-104°C |
0.6* |
Cream |
|
6 |
3A |
C19H20N6O3 |
380.4 |
87.60% |
140-142°C |
0.62** |
Light yellow |
|
7 |
3B |
C19H20N6O3 |
380.4 |
70% |
76-78°C |
0.58** |
Light yellow |
|
8 |
3C |
C21H23N5O |
349 |
86.50% |
56-58°C |
0.45** |
Dark yellow |
|
9 |
3E |
C19H20F N5O |
353.39 |
88.30% |
68-70°C |
0.35** |
Dark yellow |
Solvent used: * = n-Hexane: Ethyl acetate (6:4) ** = n-Hexane: Ethyl acetate (4:6)
Compound 2A: (2E)-1-{4-[(E)-morpholin-4-yldiazenyl] phenyl}-3-(4-nitrophenyl) prop-2-en-1-one:
Molecular Formula
C19H18N4O4, Molecular Weight: 366.37, Physical state: crystalline powder, Colour: Green, Melting Point: 216-218oC, Rf Value: 0.47 (n-hexane: Ethyl acetate; 6:4), Solubility: Chloroform, DMSO, Ethyl acetate, Percentage yield : 87%.
IR (KBr disk)
γ (cm-1), C-H Stretching (Morpholine) – 3104.94 Cm-1, C-H Stretching (Aromatic) – 2980.08 Cm-1 , C-H Stretching (olefins) – 3076.89 Cm-1, C=O – 1658.49 Cm-1 ,C=C Stretching (Olefin) – 1598.09 Cm-1 , C=C (Aromatic) – 1517.45 Cm-1, N=O (Nitro) – 1431.77 Cm-1, C-H Bending (Aromatic) – 832.65 Cm-1C-H Stretching (Morpholine) – 3104.94 Cm-1, C-H Stretching (Aromatic) – 2980.08 Cm-1 , C-H Stretching (olefins) – 3076.89 Cm-1 , C=O – 1658.49 Cm-1 ,C=C Stretching (Olefin) – 1598.09 Cm-1 , C=C (Aromatic) – 1517.45 Cm-1 , N=O (Nitro) – 1431.77 Cm-1 , C-H Bending (Aromatic) – 832.65 Cm-1 .
1H NMR (CDCl3)
δ(ppm)3.893 to 3.884 (8H,m,morpholine protons),7.575 (2H,d,CC&CE Ar-H,J=8.8Hz), 7.693 (1H,d,Ci Olefinic proton=15.6 Hz), 7.801 (2H,d,C2&C6Ar-H,J=9Hz), 7.841 (1H,d,CiiOlefinic proton=16), 8.071 (2H,d,CB&CF Ar-H,J=8.4 Hz), 8.29 (2H,d,C3&C5Ar-H,J=8.4 Hz).
Compound 2B: (2E)-1 -{4-[(E)-morpholin-4-yldiazenyl]phenyl}-3-(3-nitrophenyl)prop-2-en-1 -one
Molecular Formula
C19H18N4O4, Molecular Weight : 366.37, Physical state : crystalline powder ,Colour: Green ,Melting Point: 136-1 39oC RfValue : 0.7 (n-hexane:Ethylacetate;4:6) ,Solubility : Chloroform, DMSO, Ethyl acetate, Percentage yield : 85%.
IR (KBr disk)
γ (cm-1 ),C-H Stretching (olefins) – 3073.45 Cm-1,C-H Stretching (morpholine) – 2963.93 Cm-1,C-H Stretching (Aromatic) – 2990.21 Cm-1,C=C (Aromatic) – 1522.56 Cm-1,C-H Bending (Aromatic) – 805.79 Cm-1, C=O – 1660.04 Cm-1,N=O (Nitro) – 1437.85 Cm-1 , C=C Stretching (Olefin)- 1607.01 Cm-1
1H NMR (CDCl3)
δ(ppm)3.911 to 3.885 (8H,m,morpholine protons), 7.593 to7.558 (2H,d,CC&CE Ar-H,J=8.4Hz), 7.633 (1H,t,C5Ar-H,J=8 Hz), 7.705 (1H,d,C6Ar-H,J=8.9 Hz), 7.854 (1H,d, CiOlefinicproton,J=15.6 Hz), 7.929 (1H,d,CiiOlefinic proton,J=14 Hz), 8.084 (2H,d,CB&CF Ar-H,J=8.8Hz), 8.263 (1H,d,C4 Ar-H,J=8.4Hz), 8.518(1H,s,C6 Ar-H).
Compound 2C: (2E)-3-(4-methylphenyl)-1-{4-[(E)-morpholin-4-yldiazenyl]phenyl}prop-2-en-1-one
Molecular Formula
C20H21N3O2, Molecular Weight : 335.39, Physical state : crystalline powder, Colour:Orange,Melting Point :137-140oC,Rf Value:0.57(n-hexane:Ethylacetate;6:4) , Solubility: Chloroform, DMSO, Ethyl acetate, Percentage yield : 70%.
IR (KBr disk)
γ (cm-1),C-H Stretching (olefins) – 3052.25 Cm-1, C-H Stretching (morpholine) – 2971.19 Cm-1, C-H Stretching (Aromatic) – 2903.18 Cm-1,C-H Stretching (CH3) – 2854.95 Cm-1,C=O – 1649.71 Cm-1,C=C Stretching (Olefin) – 1588.87 Cm-1,C=C (Aromatic) – 1562.54 Cm-1,C-H Bending (Aromatic) – 810.55 Cm-1
1H NMR (CDCl3)
δ(ppm) 2.396 ( 3H,s,-CH3 protons on C4 of aldehyde), 3.876 (8H,m,morpholine protons),7.258(2H,d,CC&CEAr-H,J=8.8Hz),7.505(1H,d,Ci Olefinicproton,J=12Hz),7.560 to 7.531 (4H,m,C2,C3,C5,C6Ar-H,J=8.4Hz), 7.818(1H,d,CiiOlefinic proton,J=14.6Hz), 8.054(2H,d, CB&CF Ar-H,J=8.4 Hz).
Compound 2D :(2E)-3-(3, 4, 5-tri methoxy phenyl)-1-{4-[(E)-morpholin-4-yldiazenyl] phenyl}prop-2-en-1-one
Molecular Formula
C22H25N3O5,Molecular Weight : 411.45, Physical state: crystalline powder , Colour: Green ,Melting Point : 126-129oC,Rf Value: 0.52 (n-hexane:Ethylacetate; 4:6) Solubility : Chloroform, DMSO, Ethyl acetate. Percentage yield : 86 %.
IR (KBr disk)
γ (cm-1),C-H Stretching (olefins)-2995.03 Cm-1,C-H Stretching (morpholine)- 2973.24 Cm-1, C-H Stretching (Aromatic) – 2937.50 Cm-1,C=O – 1656.15 Cm-1, C=C (Aromatic) – 1602.99 Cm-1, C=C Stretching (Olefin) – 1502.80 Cm-1, C-O Stretching (Ether) – 1326.88 Cm-1 , C-O-C Stretching – 1248.83 Cm-1, C-H Bending (Aromatic) – 836.89 Cm-1.
1H NMR (CDCl3)
δ(ppm) 3.875 (8H,m,morpholine protons), 3.904 (3H,s,-OCH3 on C4), 3.925 (6H,s,-OCH3 on C3&C5), 6.872 (2H,s, C2&C6Ar-H), 7.453 (1H,d,Ci Olefinicproton,J=15.6Hz), 7.559 (2H,d,CC&CE Ar-H,J=8.4Hz), 7.742(1H,d,CiiOlefinicproton,J=15.6Hz), 8.052 (2H,d,CB&CF Ar-H,J=8.4Hz).
Compound 2E: (2E)-3-(4-fluorophenyl)-1-{4-[(E)-morpholin-4-yldiazenyl]phenyl}prop-2-en-1-one
Molecular Formula
C19H18FN3O2, Molecular Weight: 339.36, Physical state: Crystalline powder, Colour: Cream, Melting Point: 102-104oC,Rf Value: 0.6 (n-hexane: Ethylacetate; 6:4), Solubility: Chloroform, DMSO, Ethyl acetate, Percentage yield: 88.2%
IR (KBr disk)
γ (cm-1),C-H Stretching (olefins) – 3066.52 Cm-1,C-H Stretching (morpholine) – 2974.73 Cm-1, C-H Stretching (Aromatic) – 2866.95 Cm-1,C=O – 1656.66 Cm-1, C=C Stretching (Olefin) – 1596.46 Cm-1,C=C (Aromatic) – 1510.09 Cm-1, C-F Stretching – 1332.37 Cm-1 C-H Bending (Aromatic) – 827.50 Cm-1
1H NMR (CDCl3)
δ(ppm) 3.874 (8H,m,morpholine protons), 7.218 (2H,d,C3&C5Ar-H,J=8.4 Hz), 7.470 (1H,d,Ci Olefinicproton=15.6 Hz), 7.555 (2H,d,C2&C6Ar-H,J=8.4 Hz),7.654 to 7.619(2H,d,C3&C5Ar-H,J=8.4 Hz),7.796 (1H,d,Cii Olefinicproton=15.6Hz), 8.051 (2H,d,CB&CF Ar-H,J=8.4Hz).
Compound 3A:4-[(E)-{4-[5-(4-nitrophenyl)-4, 5-dihydro-1H-pyrazol- 3yl] phenyl} diazenyl] morpholine
Molecular Formula
C19H20N6O3, Molecular Weight : 380.4 , Physical state : Crystalline powder , Colour : Light yellow , Melting Point: 140-142oC , Rf Value : 0.35 (n-hexane:Ethylacetate; 6:4) ,Solubility : Chloroform, DMSO, Ethyl acetate, Percentage yield : 87.6%.
IR (KBr disk)
γ (cm-1),C-H Stretching (olefins) – 3355.58 Cm-1,N-H Stretching (pyrazoline) – 3105.16 Cm-1, C-H Stretching (morpholine) – 3072.35 Cm-1, C-H Stretching (Aromatic) – 2953.16 Cm-1, N=O (Nitro) – 1598.61 Cm-1, C=C (Aromatic) – 1513.25 Cm-1, C=N Stretching (Pyrazoline) – 1437.94 Cm-1, C5-N1 Stretching (Pyrazoline)- 1156.89 Cm-1, C-H Bending (Aromatic) – 843.41 Cm-1.
1H NMR (CDCl3): δ(ppm)
3.035 to 2.970 (1H,dd,Hb on C4 of pyrazoline ring), 3.611 to 3.544 (1H,dd,Ha on C4 of pyrazoline ring), 3.863 to 3.819 (8H,m,morpholine protons), 5.069 to 5.018 (1H,m, Hx on C5 of pyrazoline ring), 6.080 (1H,d,N1H proton in pyrazoline ring), 7.264 (2H,d,C2&C6Ar-H,J=8.4Hz), 7.657 to 7.579 (4H,m, CB,CF, CC&CE Ar-H,J=8.4 Hz), 8.225 (2H,d,C3&C5Ar-H,J=8.Hz), Jab=16.4, Jax=5.2, Jbx =10.8.
Compound 3B:4-[(E)-{4-[5-(3-nitrophenyl)-4, 5-dihydro-1H- pyrazol3yl] phenyl}diazenyl]morpholine
Molecular Formula
C19H20N6O3,Molecular Weight : 380.4 ,Physical state : Crystalline powder ,Colour : Light yellow ,Melting Point: 76-78oC ,Rf Value : 0.58 (n-hexane:Ethylacetate; 4:6) ,Solubility: Chloroform, DMSO, Ethyl acetate, Percentage yield : 70%
IR (KBr disk)
γ (cm-1),C-H Stretching (olefins)-3336.76 Cm-1, C-H Stretching (morpholine) – 3308.19 Cm-1, N-H Stretching (pyrazoline) – 3085.54 Cm-1, C-H Stretching (Aromatic) – 2857.31 Cm-1, C=C (Aromatic) – 1438.17 Cm-1, N=O (Nitro) – 1524.22 Cm-1, C=N Stretching (Pyrazoline) – 1348.84 Cm-1, C5-N1 Stretching (Pyrazoline)- 1154.79 Cm-1, C-H Bending (Aromatic) – 835.42 Cm-1.
1H NMR (CDCl3): δ(ppm) 3.050 to 2.985 (1H,dd,Ha on C4 of pyrazoline ring), 3.617 to 3.577 (1H,dd,Hb on C4 of pyrazoline ring), 3.857 to 3.550 (8H,m,morpholine protons), 5.083 to 5.032 (1H,m, Hx on C5 of pyrazoline ring), 7.481 to 7.460 (2H,d, CC&CE Ar-H,J=8.4 Hz), 7.557 to 7.518 (2H,d, C4&C6 Ar-H,J=8Hz), 7.662 to 7.642 (2H,d, CB&CF Ar-H,J=8 Hz), 7.789 (1H,d,N1H proton in pyrazoline ring), 8.163 (1H,t, C5 Ar-H,J=8.1Hz), 8.284 (1H,s, C2 Ar-H), Jab=16 , Jax=6 , Jbx =10.8.
Compound 3C:4-[(E)-{4-[5-(4-methylphenyl)-4, 5-dihydro-1H-pyrazol-3-yl] phenyl}diazenyl]morpholine
Molecular Formula:C21H23N5O,Molecular Weight : 349, Physical state : Crystalline powder , Colour : Dark yellow , Melting Point : 56-58oC , Rf Value : 0.45 (n-hexane:Ethylacetate; 6:4) ,Solubility : Chloroform, DMSO, Ethyl acetate. Percentage yield : 86.5%
IR (KBr disk)
γ (cm-1),C-H Stretching (olefins)-3355.61 Cm-1,C-H Stretching (morpholine) – 3333.83 Cm-1, N-H Stretching (pyrazoline) – 3025.31 Cm-1, C-H Stretching (Aromatic) – 2918.99 Cm-1, C-H Stretching (CH3) – 2854.54 Cm-1, C=C (Aromatic) – 1435.06 Cm-1, C=N Stretching (Pyrazoline) – 1348.57 Cm-1. C5-N1 Stretching (Pyrazoline) – 1154.81 Cm-1, C-H Bending (Aromatic) – 814.53 Cm-1
1H NMR (CDCl3): δ(ppm)
2.391 (3H,s,CH3 on C4), 3.072 to 3.010 (1H,dd, Ha on C4 of pyrazoline ring), 3.487 to 3.420 (1H,dd,Hb on C4 of pyrazoline ring), 3.815 (1H,m, Hx on C5 of pyrazoline ring), 3.855 (8H,m,morpholine protons), 7.192 (1H,d,N1H proton in pyrazoline ring), 7.530 to 7.448 (4H,d, C2,C3, C5&C6 Ar-H,J=8.4 Hz), 7.665 to 7.595 (4H,d, CB,CF, CC&CE Ar-H,J=8.4 Hz), Jab=16.4 , Jax=6.4 , Jbx =10.4.
Compound 3E:4-[(E)-{4-[5-(4-fluorophenyl)-4, 5-dihydro-1H-pyrazol-3-yl] phenyl} diazenyl] morpholine
Molecular Formula:C19H20FN5O, Molecular Weight : 353.39, Physical state : Crystalline powder , Colour : Dark yellow , Melting Point : 68-70oC ,Rf Value: 0.35 (n-hexane:Ethyl acetate; 6:4) , Solubility : Chloroform, DMSO, Ethyl acetate, Percentage yield : 88.3%.
IR (KBr disk)
γ (cm-1),C-H Stretching (olefins) – 3063.27 Cm-1, C-H Stretching (morpholine) – 3036.41 Cm-1, N-H Stretching (pyrazoline) – 2967.44 Cm-1,C-H Stretching (Aromatic) – 2855.66 Cm-1, C=C (Aromatic) – 1438.58 Cm-1, C=N Stretching (Pyrazoline) – 1347.59 Cm-1, C5-N1 Stretching (Pyrazoline)- 1156.12 Cm-1, C-F Stretching – 1015.78 Cm-1, C-H Bending (Aromatic) – 839.10 Cm-1.
1H NMR (CDCl3): δ(ppm)
3.040 to 2.976 (1H,dd,Ha on C4 of pyrazoline ring), 3.510 to 3.442 (1H,dd,Hb on C4 of pyrazoline ring), 3.868 to 3.800 (8H,m,morpholine protons), 4.942 to 4.483 (1H,m, Hx on C5 of pyrazoline ring), 7.050 to 7.007 (2H,d, CC&CE Ar-H,J=8.8 Hz),7.374 (1H,d,N1H proton in pyrazoline ring), 7.476 to 7.454 (4H,d, C2,C3, C5&C6 Ar-H,J=8.8 Hz), 7.664 to 7.643 (2H,d, CB&CF Ar-H,J=8.4 Hz), Jab=16.4 , Jax=6.8 , Jbx =10.8.
Table 2: The percentage scavenging activity by DPPH Method
| Concμg/ml |
% scavenging activity(Mean ± SEM) |
|||||||||
| Std | 2A | 2B | 2C | 2D | 2E | 3A | 3B | 3C | 3E | |
| 20 | 84.26 ±0.176 | 49.29±0.159 | 45.43±0.088 | 58.43±0.296 | 63.50±0.288 | 55.50±0.288 | 50.30±0.650 | 44.46±0.290 | 58.73±0.218 | 46.40±0.305 |
| 40 | 85.40±0.208 | 50.26±0.091 | 49.35±0.224 | 64.60±0.305 | 67.90±0.450 | 58.73±0.371 | 51.560.808 | 49.33±0.333 | 61.33±0.333 | 49.96±0.983 |
| 60 | 86.36±0.185 | 53.63±0.120 | 50.46±0.218 | 67.50±0.288 | 70.40±0.305 | 62.06±0.560 | 55.800.416 | 52.60±0.264 | 65.86±0.466 | 54.53±0.290 |
| 80 | 89.30±0.152 | 56.53±0.088 | 55.43±0.120 | 71.66±0.240 | 73.63±0.272 | 64.60±0.305 | 58.730.371 | 55.80±0.416 | 71.93±0.520 | 60.70±0.351 |
| 100 | 90.40±0.115 | 61.33±0.145 | 60.63±0.272 | 72.06±0.581 | 76.60±0.305 | 65.86±0.466 | 64.660.240 | 61.67±0.338 | 74.96±0.548 | 65.93±0.405 |
| 120 | 91.33±0.176 | 66.40±0.115 | 62.83±0.120 | 74.60±0.305 | 78.86±0.466 | 69.13±0.592 | 66.830.440 | 65.30±0.208 | 81.63±0.317 | 69.16±0.088 |
| IC50μg/ml | 2.80 | 58.61 | 64.20 | 42.81 | 36.30 | 50.62 | 55.42 | 60.02 | 32.34 | 54.90 |
Mean ± SEM = Mean ± Standard Error Mean, IC50=Half maximal inhibitory concentration
Table 3: The percentage scavenging activity by Hydrogen Peroxide Method
|
Conc |
% scavenging activity(Mean ± SEM) |
|||||||||
|
μg/ml |
Std |
2A |
2B |
2C |
2D |
2E |
3A |
3B |
3C |
3E |
|
20 |
89.7 |
44.53 |
33.46 |
48.7 |
50.2 |
52.6 |
47.9 |
37.83 |
55.6 |
46.4 |
|
±0.351 |
±0.290 |
±0.290 |
±0.351 |
±0.099 |
±0.305 |
±0.493 |
±0.440 |
±0.305 |
±0.305 |
|
|
40 |
92.6 |
46.86 |
35.06 |
51.36 |
55.2 |
57.767 |
49.6 |
40.76 |
61.86 |
58.733 |
|
±0.305 |
±0.466 |
±0.066 |
±0.317 |
±0.416 |
±0.39 |
±0.305 |
±0.145 |
±0.466 |
±0.371 |
|
|
60 |
94.46 |
50.23 |
37.73 |
54.86 |
59.4 |
59.267 |
52.6 |
42.36 |
63.63 |
60.53 |
|
±0.240 |
±0.392 |
±0.371 |
±0.466 |
±0.305 |
±0.266 |
±0.305 |
±0.317 |
±0.317 |
±0.290 |
|
|
80 |
95.06 |
53.76 |
42.7 |
58.7 |
61.36 |
61.567 |
58.23 |
45.63 |
67.46 |
62.23 |
|
±0.066 |
±0.392 |
±0.351 |
±0.351 |
±0.317 |
±0.296 |
±0.120 |
±0.3179 |
±0.290 |
±0.185 |
|
|
100 |
95.36 |
55.83 |
47.6 |
60.4 |
64.93 |
63.267 |
59.03 |
47.26 |
70.63 |
62.8 |
|
±0.185 |
±0.440 |
±0.305 |
±0.305 |
±0.520 |
±0.133 |
±0.088 |
±0.176 |
±0.202 |
±0.115 |
|
|
120 |
96.2 |
57.83 |
49.53 |
63.43 |
69.4 |
64.4 |
60.5 |
48.56 |
72.6 |
65.56 |
|
±0.100 |
±0.440 |
±0.290 |
±0.296 |
±0.305 |
±0.305 |
±0.288 |
±0.296 |
±0.305 |
±0.296 |
|
|
IC50 |
2.2 |
62.41 |
116.02 |
24.23 |
20.18 |
32.41 |
50.11 |
120.06 |
16.28 |
40.92 |
|
μg/ml |
||||||||||
Mean ± SEM = Mean ± Standard Error Mean, IC50=Half maximal inhibitory concentration
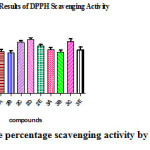 |
Figure 4: The percentage scavenging activity by DPPH Method Click here to View figure |
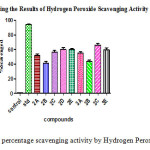 |
Figure 5: The percentage scavenging activity by Hydrogen Peroxide Method Click here to View figure |
Table 4: The percentage scavenging activity by Egg Albumin Denaturation Method
|
Conc |
% Inhibition(Mean ± SEM) |
|||||||||
|
μg/ml |
Std |
2A |
2B |
2C |
2D |
2E |
3A |
3B |
3C |
3E |
|
20 |
76.83 ±0.441 |
25.86 ±0.592 |
32.56 ±0.260 |
36.40 ±0.305 |
33.4 |
30.6 |
28.4 |
23.8 |
40.7 |
29.93 |
|
±0.305 |
±0.305 |
±0.305 |
±0.416 |
±0.351 |
±0.520 |
|||||
|
40 |
78.2 ±0.115 |
30.46 ±0.290 |
34.26 ±0.176 |
37.30 ±0.251 |
35.7 |
41.63 |
33.46 |
35.46 |
42.2 |
31.66 |
|
±0.152 |
±0.318 |
±0.290 |
±0.290 |
±0.200 |
±0.176 |
|||||
|
60 |
79.30 ±0.173 |
36.63 ±0.318 |
37.36 ±0.318 |
43.26 ±0.176 |
40.2 |
43.56 |
41.63 |
42.5 |
45.5 |
33.26 |
|
±0.416 |
±0.296 |
±0.318 |
±0.288 |
±0.288 |
±0.176 |
|||||
|
80 |
80.06 ±0.066 |
43.46 ±0.290 |
43.33 |
48.43 ±0.233 |
47.4 |
45.16 |
43.7 |
44.36 |
47.5 |
35.33 |
|
±0.166 |
±0.305 |
±0.088 |
±0.351 |
±0.272 |
±0.288 |
±0.176 |
||||
|
100 |
81.40 ±0.115 |
46.40 ±0.305 |
44.53 ±0.290 |
51.46 ±0.290 |
55.56 |
46.56 |
46.4 |
45.26 |
52.36 |
48.46 |
|
±0.296 |
±0.296 |
±0.305 |
±0.176 |
±0.318 |
±0.290 |
|||||
|
120 |
82.63 ±0.202 |
51.30 ±0.351 |
47.40 ±0.305 |
56.73 ±0.371 |
61.8 |
54.3 |
51.63 |
46.6 |
58.53 |
53.73 |
|
±0.416 |
±0.351 |
±0.318 |
±0.305 |
±0.290 |
±0.371 |
|||||
|
IC50 |
1.02 |
116.32 |
134.04 |
86.43 |
84.22 |
104.08 |
114.02 |
128.4 |
82.06 |
106.28 |
|
μg/ml |
||||||||||
Mean ± SEM = Mean ± Standard Error Mean, IC50=Half maximal inhibitory concentration
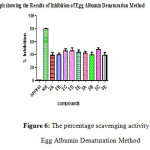 |
Figure 6: The percentage scavenging activity by Egg Albumin Denaturation Method Click here to View figure |
Table 5: The percentage scavenging activity by Egg Albumin Denaturation Method
|
Conc |
% Inhibition(Mean ± SEM) |
|||||||||
|
μg/ml |
Std |
2A |
2B |
2C |
2D |
2E |
3A |
3B |
3C |
3E |
|
20 |
53.36 |
29.6 |
27.56 |
35.83 |
33.7 |
31.2 |
31.9 |
30.43 |
35.5 |
34.23 |
|
±0.318 |
±0.305 |
±0.296 |
±0.441 |
±0.351 |
±0.115 |
±0.435 |
±0.296 |
±0.288 |
±0.145 |
|
|
40 |
62.4 |
31.53 |
35.76 |
40.53 |
35.86 |
32.63 |
34.7 |
32.26 |
38.63 |
35.36 |
|
±0.305 |
±0.290 |
±0.393 |
±0.290 |
±0.466 |
±0.318 |
±0.251 |
±0.218 |
±0.318 |
±0.318 |
|
|
60 |
67.86 |
35.43 |
37.7 |
48.93 |
37.56 |
34.36 |
36.86 |
34.6 |
40.46 |
37.7 |
|
±0.466 |
±0.296 |
±0.351 |
±0.520 |
±0.296 |
±0.318 |
±0.466 |
±0.305 |
±0.290 |
±0.351 |
|
|
80 |
71.9 |
42.46 |
40.46 |
50.2 |
42.2 |
37.86 |
39.36 |
37.5 |
41.53 |
40.4 |
|
±0.493 |
±0.290 |
±0.290 |
±0.200 |
±0.611 |
±0.466 |
±0.318 |
±0.288 |
±0.290 |
±0.305 |
|
|
100 |
74.6 |
44.2 |
42.26 |
51.16 |
49.46 |
42.6 |
43.46 |
43.73 |
45.6 |
42.46 |
|
±0.305 |
±0.200 |
±0.176 |
±0.088 |
±0.290 |
±0.305 |
±0.290 |
±0.371 |
±0.305 |
±0.240 |
|
|
120 |
75.56 |
45.86 |
44.4 |
52.46 |
54.8 |
48.33 |
49.86 |
48.53 |
55.16 |
50.66 |
|
±0.296 |
±0.466 |
±0.305 |
±0.290 |
±0.416 |
±0.333 |
±0.240 |
±0.290 |
±0.166 |
±0.176 |
|
|
IC50 |
13.02 |
128.01 |
174.51 |
90.2 |
102.01 |
126.04 |
130.45 |
142.05 |
100 |
164.23 |
|
μg/ml |
||||||||||
Mean ± SEM = Mean ± Standard Error Mean, IC50=Half maximal inhibitory concentration
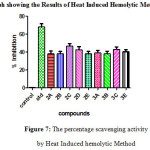 |
Figure 7: The percentage scavenging activity by Heat Induced hemolytic Method Click here to View figure |
The data was analyzed by one way ANOVA using Graph pad prism software. The scavenging activity of the compounds ( Table 2&3) and their IC50 values were compared with the standard and the significance factor “p” was found to be less than 0.001 for most of the compounds. Among chalcones compound 2D having 3,4,5- trimethoxy substitution and among pyrazolines compound 3C having 4-Methyl has shown highest activity with least IC50 values suggesting that electron donating groups aid in scavenging activity. Among chalcones compound 2B and among pyrazolines compound 3B having 3-Nitro substitutions has shown least activity with highest IC50values indicating that electron withdrawing groups at the meta position in the compounds have less scavenging activity. All the other compounds with 4-Nitro, 4- fluorosubstituents were found to have intermediate activity.
Similarly the anti inflammatory activity of the compounds and their IC50 values were compared with the standard and the significance factor p≤ 0.001 for most of the compounds ( Table 4 & 5). Among chalcones compound 2D having 3,4,5- trimethoxy substitution and among pyrazolines compound 3C having 4-Methyl has shown highest activity with least IC50values suggesting that electron donating groups in the compounds aid in good anti inflammatory activity. Among chalcones compound 2B and among pyrazolines compound 3B having 3-Nitro substitutions with highest IC50values has shown least activity indicating that electron withdrawing groups at the meta position in the compounds have less anti inflammatory activity. All the other compounds with 4-Nitro, 4- fluoro substituents were found to have intermediate activity.
Conclusion
4- Amino acetophenone was diazotized then followed by coupling with with morpholine as ameans of protection of amine group in 4-Amino acetophenone. The obtained diazotizedproduct containing the acetyl group is then made to react with different aldehydes in the presence of 40% KOH solution as catalyst to get chalcone derivatives. The sechalcone derivatives are then subjected for cyclization by treating with hydrazine hydrate. All the reactions were monitored by TLC to ascertain the completion of the reaction. All the compoundswere found to have good yields. Rf values and melting points of the synthesized compounds were different with each other indicating the difference between the compounds. The structures were proposed based on1H NMR and IR spectral data.
Anti oxidant activity was performed for the synthesized compounds by DPPH and Hydrogen Peroxide Method, and anti inflammatory activity was performed by Inhibition Of Egg Albumin Denaturation Method and Heat Induced Haemolytic Method. Most of the compounds showed significant activity with p≤ 0.001 when the data was subjected to one way ANOVA by using Graph pad prism-5 software. Among chalcones compound 2D having 3,4,5- trimethoxy substitution and among pyrazolines compound 3C having 4-Methyl has shown highest activity with least IC50values which is considerable with the standard, suggesting that electron donating groups aid in scavenging activity. Among chalcones compound 2B and among pyrazolines compound 3B having 3-Nitro substitutions has shown least activity with highest IC50 values which is considerable with the standard, suggesting that electron withdrawing groups at the meta position in the compounds have less scavenging activity. All the other compounds with 4-Nitro, 4- fluorosubstituent’s were found to have intermediate activity.
Acknowledgement
The authors are thankful to the Principal, Creative Educational Society’s College of pharmacy for providing the necessary facilities to carry out the research.The authors are also thankful to Dr.Murugesan, SAIF, IIT-Chennai for providing1H NMR data.
References
- Zhao, Y.L.; Chen, Y.L.; Chang, F.S.; Tzeng, C.C. E J Med Chem. 2005, 40, 792–797
- Singh, V.; Argal, A.; Mishra, V.; Raghuvanshi, R.; Agnihotri, S. Int J Res Pharm Sci. 2011, 1(3), 125-146
- Kalirajan, R.; Rafick, M. H.;Sankar, S.;Jubie , S. Sci World J.2012, 3, 1-15
- Kathiriya, P. J.; Purohit, D. M. J Chem& Pharm Res.2012, 4(1), 383-386
- Song, D.M.; Jung, K.H.; Moon, J.H.; Shin, D.M.Optical Materials.2002, 21, 667–671
- Karale B. K.;Takate S. J.; Salve S. P.; Zaware B. H.; Jadhav S. S. Orient J Chem.2015;31(1),.307-315
- Jagadhani S. G.; Kundlikar S. G.; Karale B. K. Orient J Chem.2015; 31(1),.601-604
- Ozdemir, A.; Zitouni, G.T.; Kaplancikli, Z.A.Turk J Chem.2008, 32, 529 – 538
- Sarkar ,B.K.; Patel,R.;Bhadoriya,U.J Adv Pharm Edu& Res. 2011, 1(5), 243-250
- Vani, T.;Rajani, M.; Sarkar,S.;Shishoo, C.J. Int. J. Pharmacognosy.1997, 35, 313-317
- Navarro, M.C.Plantamedica.1993, 59, 312-314
- Chandra, S.; Priyanka, C.; Protapaditya, D.; Bhattacharya, S. Asian Pacific J Tropical Biomed.2012, 7 (4), 178-180
- Chaitanya,S. C.;Meena, V. JChem Bio&Phy Sci.2011, 3, 2249-2253
- Cheng, Y.;Prusoff, W.H.Biochem Pharmacol.1973, 22(23), 99–108.

This work is licensed under a Creative Commons Attribution 4.0 International License.









