Deuterium adsorption on Multi Carbon Nano-cone (MNCx, X=2-7) including BN Nano-cone: A model for D2 storage
Sepideh Moradi*
Department of chemistry, Science and research branch, Islamic Azad University, Tehran, Iran Corresponding Author: sepideh.mm92@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310313
Article Received on :
Article Accepted on :
Article Published : 29 Jul 2015
A mixing of Multi Wall (BN&C) Nano-cones have been modeled and calculated for the suitable structures to storage the Deuterium molecules. We have found these kinds of nano-structures are useful for maximum storages of D2 molecules. B3LYP/6-31G/6-31G/6-31G*/6-31+G* density functional theory (DFT) calculations have performed for the structure and stability of two, three, four and seven wall (BN&C) Nano-cones. In this work, it was calculated the geometrical structure, and stability to predict NMR and NBO parameters.
KEYWORDS:storages of D2 molecule; Density functional theory (DFT); Ab-initio calculation; Thermodynamic parameters
Download this article as:| Copy the following to cite this article: Moradi S. Deuterium adsorption on Multi Carbon Nano-cone (MNCx, X=2-7) including BN Nano-cone: A model for D2 storage. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Moradi S. Deuterium adsorption on Multi Carbon Nano-cone (MNCx, X=2-7) including BN Nano-cone: A model for D2 storage. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10096 |
Introduction
Deuterium is one of two stable isotopes of hydrogen. The nucleus of deuterium, called a deuteron, contains one proton and one neutron, whereas the far more common hydrogen isotope, protium, has no neutron in the nucleus.
Deuterium is destroyed in the interiors of stars faster than it is produced. Other natural processes are thought to produce only an insignificant amount of deuterium. Theoretically nearly all deuterium found in nature was produced in the Big Bang 13.8 billion years ago, as the basic or primordial ratio of hydrogen-1 (protium) to deuterium (about 26 atoms of deuterium per million hydrogen atoms) has its origin from that time. This is the ratio found in the gas giant planets, such as Jupiter [1-3].
Although much study has been done for nanotube carbon phenomenon [4-12], there are a few studies for Multi Nano-cone. The carbon nanotube (CNT) is a representative nano-material. CNT is a cylindrically shaped carbon material with a nano-metric-level diameter [4-17].
Carbon nano cone has a high asymmetric geometry that in our simulations, classical non-equilibrium molecular dynamics method is adopted. The cone is entirely characterized by its cone angle. When one pentagon is introduced into a hexagonal carbon network, a 60 declination defect is formed; leading to the formation of a nano cone with cone angle of 118 and the equilibrium carbon-carbon bond length is 1.418 Å. In this work, we focus on the cone with the cone angle of 180, which is the largest angle observed experimentally and theoretically. Moreover, in all theoretical models so far, the rectification efficiency
decreases quickly as the structure length increases.
Its structure, which is in the form of a hexagonal mesh, resembles a graphite sheet and it carries a carbon atom located on the vertex of each mesh. The sheet has rolled and its two edges have connected seamlessly [5-22].
Although it is a commonplace material using in pencil leads, its unique structure causes it to present characteristics that had not found with any other materials. CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite [10-25].
The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers. The length is usually in the order of microns, but single-wall CNT with a length in the order of centimeters has recently released [20-35].
CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite. The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers [30-40].
The length is usually in the order of microns, but single-wall CNT with a length about centimeters have recently released. The extremities of the CNT have usually closed with lids of the graphite sheet [37-45].
The lids consist of hexagonal crystalline structures (six-membered ring structures) and a total of six pentagonal structures (five-membered ring structures) placed here and there in the hexagonal structure [40-50]. The first report by Iijima was on the multi wall form, coaxial carbon cylinders with a few tens of nanometers in outer diameter. Two years later, up to now, single walled nanotubes were reported in various works [5-20]. SWCNTs have considered as the leading candidate for nano-device applications because of their one-dimensional electronic bond structure, molecular size, and biocompatibility, controllable property of conducting electrical current and reversible response to biological reagents hence SWCNTs make possible bonding to polymers and biological systems such as DNA and carbohydrates [48-55].
boron nitride nanotube (BNNTs) have attracted much interests due to their large gap semi conducting character[49-60].Boron nitride (BN) is a structural existing in cubic (diamond-like), hexagonal (graphite-like), turbo static, and amorphous forms .these compounds have been produced by a variety of methods, such as arc melting[50-59], high temperature chemical reaction[54-64], carbon nanotube templates[50-66], and laser ablating[59-69] The most attention has been focused on the development of new methods for the production of nanotube and inorganic fullerene of other materials.
In addition, theoretical calculations have been described the possible existence of small BN clusters [61-77].
Theoretical studies have been performed for BN doped in CNTs which it has been found that a structure built from squares and hexagons is more stable than those built from pentagons and hexagons. This is because in the second case less stable B-B and N-N bonds are formed, [65-85].
The most stable TWC-Nano-cones structure is built from. [75-104]
In this work, we have focused on MWC-Nano-cones and double Wall C -(BN) nano-con. Our aim was to obtain the global minimum energy structure. For this structure, we use the QM/MM methods of hybrid B3LYP exchange-correlation functional within density functional theory. Primary, structure optimization calculated and then Nuclear Magnetic Resonance (NMR) parameters by density Functional Theory (DFT) method calculated on the optimized structure. Isotropic chemical shielding, anisotropic chemical shielding parameters at all of the atoms nuclei are presented in Table 1. And also, Thermodynamic Properties have been considered in Table 2
 |
Table1: HOMO and LUMO and Gap energy of Nano-cone including 7 layers (between, atoms 520 – 560) Click here to View table |
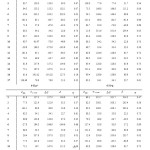 |
Table2: NMR Parameters of Swbnnts @ Swcnts Click here to View table |
We have found that these kinds of Nano-tubes are useful for H2 Storage. In material sciences Boron nitride, which appears in a manifold of crystalline modifications, has been an extremely practical material with hexagonal and cubic boron nitride as most outstanding materials (for doping). The BN cluster is a polar molecule and BN doped in nanotubes have an inert chemical structure. We can see that there is a negative charge at nitrogen atom and a positive charge at boron atom, so we can use an electrophilic or nucleophilic reagent as a solution for BN clusters.
BN nanotubes are very suitable for composite materials because these structures have a higher temperature resistance to oxidation than the carbon nanotubes. All the BN nanotubes are semiconductors. The BN doped in nanotubes have the band gaps which can be greater than 2 eV for most tubes also we know that the smallest carbon nanotubes are semiconductor and these structures obtain the properties of graphite when the diameter of these structures increases but BN nanotubes are semiconductors without attention to the diameter. On the basis of the similarities in characteristics between carbon and BN-based (BN=boron nitride) substances, BN-based nanotubes can be stable and therefore their electronic structure can be studied. The comparison between BN nanotubes and carbon nanotubes shows that BN nanotubes have more interesting characteristics for doping in carbon nanotubes [60-100].
Recently the mixing of boron nitride (BNNTs) and (CNTs) in a nanoscale particles have been investigated and these structures are made up of conical shells without any seamless. Most of the studies about these compounds have been done so far with carbon structures. [55-104].
Considering the above mentioned, (BN-C) NTs nanotubes are very important and interesting for new research, especially for H2 storage and can open a huge spectrum in the field of theoretical and experimental research. In the fig.1 structure of MWC-Nano-conesis shown and this particular nanometer configuration has been proposed in this research [85-106].
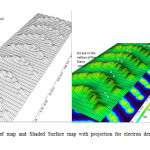 |
Figure1: Relief map and Shaded Surface map with projection for electron density of 7 Nanocone. Click here to View figure |
Computational Method
In this work, the chemical shielding is built by a three-by-three matrix which is biodegraded into a single scalar term, three antisymmetric pseudo vector components, and five components which correspond to a symmetric tensor. It can be observed the single scalar and the five symmetric tensor elements in the normal NMR spectra of the solids.
DFT (density functional theory) is one of the computational methods which can be used in different systems and it is more useful for some calculations than other methods. It is clear that basis sets are vast various.
The Gaussian 98 program was run to obtain the best prediction of this particular structure. Also all Ab-Initio and DFT (density functional theory) calculations were done with the Gaussian 98 program. Frequency analyses were carried out to show that the optimized structures are true minima or transition states on the potential energy surfaces of a specific structure without imaginary frequencies. geometry optimizations in the gas phase for MWC-Nano-cones were performed at density functional theory (DFT) level with B3LYP and Ab-Initio with HF (hartree fock) methods in different basis sets at the temperature of 298.15 K, The parameters were calculated for MWC-Nano-cones in the gas phase in different methods and basis sets include thermodynamic and NMR parameters. The chemical shielding shows the phenomenon which is dependent on the secondary magnetic field which is built by the induced movements of the electrons which encompass the nuclei.
The chemical shielding tensor includes the chemical shift isotropy (CSI) and chemical shift anisotropy (CSA) and the anisotropy (Δб) of the tensor, the shielding tensor asymmetry parameter (η) and chemical shift (δ) are calculated.
The chemical shift refers to phenomenon which associated with the secondary magnetic field created by the induced motions of the electrons that surrounding the nuclei when in the presence of an applied magnetic field. .. The shielding σ, is the differential resonance shift due to the induced motion of the electrons. The chemical shielding tensor is commonly referred to the chemical shift anisotropy (CSA) tensor according to the possession of second rank properties. The CSA tensor can be described by three additional parameters.
a) The isotropic value(σiso), of the shielding tensor which can be defined as: [59-61].
![]()
b) The chemical shift anisotropy parameter (∆σ), due to the following expression:
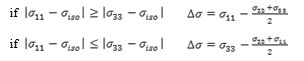
and
c) The asymmetry parameter (ƞ), which has given by:
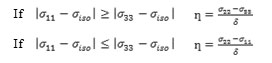
It is useful to define the span (Ω), and the skew (ĸ) of a CSA tensor. The span is defined as:
Ω = σ33 -σ 11
And indicates the width of the NMR line shape for a nonspinning, stationary, sample . The skew is defined as:
![]()
Results and Discussion
The results are listed in tables 1-3, and the figures are explained in figs 1-4. The geometry optimization for Multi Nano-cone (MNCx, X=2-7) including BN doping have been done with HF and B3LYP methods at different basis sets such as 4-31G, 6-31G, 6-31G* and 6-31+G*. Then thermodynamic properties were calculated for this structure in gas phase at 298.15K in the same methods and basis sets.
HOMO and LUMO and Gap energy of MWC-Nano-cones (atoms between, 520-560) are listed in Table1.
Relief map and Shaded Surface map with projection for electron density of 7 Nano-cone of Multi Nano-cone (MNCx, X=2-7) are shown in Fig1.
Out Put and plot of density electron from Atoms 1-560 are shown in Fig2. Localized Orbital Locator (LOL)@Electron Localization Function ELF of Multi Nano-cone (MNCx, X=2-7) including BN doping are shown in Fig1.
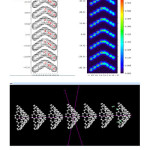 |
Figure2: The electron density distribution of 7 Nano-cone Click here to View figure |
The situation of Deuterium adsorption between two nano cones and The situation of Deuterium adsorption between two nano cones 1-BN 2-Carbon are shown in Fig3 and Fig4 .Considering the optimized structure, the NMR shielding tensors were calculated then these parameters were used to show active sites in this structure. The results of бiso, бaniso, δ, Δб and η for this nanocone in the same methods and basis sets are shown in table 3.Finally the charts of бiso, бaniso, δ and η for the atoms of Multi Nano-cone (MNCx, X=2-7) including BN doping in the 4-31G, 6-31G, 6-31G*, 6-31+G* level of theory and B3LYP and HF methods. We can obtain the interesting results from the NMR charts. Comparison of these charts (бiso, бaniso, δ and η) shows that some of peaks in these charts are similar to each other. If these peaks are reviewed, we can understand which similar atoms are situated in the same peaks of different charts. The comparison of these peaks shows that three atoms are exactly repeated in бiso, бaniso, δ and η charts. These three atoms are the active sites in this structure in Multi Nano-cone (MNCx, X=2-7) including BN doping. In general, the chart of electronic charge in different methods and basis sets is similar to the charts of NMR parameters Nitrogen atoms have more electrons than Boron atoms therefore the location of negative electronic charge is on Nitrogen atoms and positive electronic charge is situated on Boron atoms. It is clear that Nitrogen atoms will be active sites in this structure.
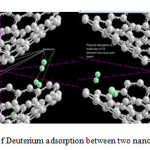 |
Figure3: The situation of Deuterium adsorption between two nano cones Click here to View figure |
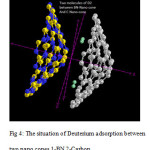 |
Figure4: The situation of Deuterium adsorption between two nano cones 1-BN 2-Carbon Click here to View figure |
Conclusion
In summary, the stability of MWC-Nano-cones and SWBNNTs @ DWCNTs were investigated. It is found that the amount of Gibbs free energy (G) , Enthalpy (H) and internal Energy (E) obtained in B3LYP/6-31+G* level in the gas phase ( 298.15K ) are the largest amount and also optimization of MWC-Nano-cones and SWBNNTs @ DWCNTs at the B3LYP/6-31+G*is suitable for this structure. The NMR data and the thermodynamics results indicate that this kind of nano-structures is suitable for H2 Storage.
References
- Hartogh, Paul; Lis, Dariusz C.; Bockelée-Morvan, Dominique; De Val-Borro, Miguel; Biver, Nicolas; Küppers, Michael; Emprechtinger, Martin; Bergin, Edwin A. et al. Nature. 2011, 478 (7368), 218–220.
- Hersant, Franck; Gautier, Daniel; Hure, Jean‐Marc. The Astrophysical Journal, 2001, 554 (1), 391–407.
- Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J. J. et al.. Science, 2014, 347: 1261952..
- Rubio Angel; Corkill , Jennifer L.; Cohen , Marvin L. Phys. Rev. 1994. B, 49, 5081
- Bourgeois,L.; Bando,Y.; Han, W.Q.; Sato, T.Phys. Rev. 2000, B, 61, 7686.
- Terauchi, M.; Tanaka, K.; Suzuki, A.; Ogino, K.; Kimura, Chem. Phys. Lett. 2000, 324, 359.
- Sachdeva, H, Frank Müllera, F .; Stefan Hüfnerb, S. Diamond and Related Materials, 2010. 19, 1027-1033.
- Massimo, Fusaro .:Quantum Matter,2014, 3, 481-487
- Micheal Arockiaraj , Rev. Theor. Sci. 2014, 2, 261-273
- Kroto, H. W.; Health, J. R.; O’Brien, S. C.; Curl, R. F.; Smalley, R. E. C60: Buckminsterfullerene. Nature (London), 1985, 318, 162
- Iijima Sumio. Nature (London), 1991. 354, 56.
- Chopra, Nasreen G.; Luyken , R. J.; Cherrey , K.; Crespi , Vincent H.; Cohen, Marvin L.; Louie, Steven G.; Zettl , A. Science,1995, 269, 966.
- Monajjemi, M.; Baei, M.T.; Mollaamin, F. Russian Journal of Inorganic Chemistry. 2008, 53 (9), 1430-1437
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
- Monajjemi, M.; Seyed Hosseini, M. Journal of Computational and Theoretical Nanoscience .2013 ,10 (10), 2473-2477
- Yahyaei ,H.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures.2014, 22(4), 346–361
- Monajjemi, M .; Jafari Azan, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures.2013, 21(6), 503–515
- Bhupesh, Bishnoi .; Bahniman ,Ghosh .Quantum Matter.2014, 3, 469-475
- Sule, Celasun , Rev. Theor. Sci.2013, 1, 319-343
- Akshaykumar, Salimath .; Bahniman, Ghosh, Quantum Matter, 2014, 3, 72-77
- Nafisi, S.; Monajemi, M.; Ebrahimi, S. Journal of Molecular Structure. 2004,705 (1-3) 35-39
- Monajjemi , M.; Baheri ,H.; Mollaamin ,F. Journal of Structural Chemistry.2011 52(1), 54-59
- Monajjemi, M.; Seyed Hosseini, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21, 381–393
- Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013, 117, 1670 −1684
- Davide Fiscaletti and Amrit Sorli ,Quantum Matter,2014 3, 200-214
- Monajjemi , M.; Honaparvar , B.; Khalili Hadad ,B.; Ilkhani ,AR.; Mollaamin, F. Afr. J. Pharm. Pharmacol .2010, 4 (8), 521-529
- Bjürn Piglosiewicz, Jan Vogelsang.;Slawa Schmidt.; Doo Jae Park.; Petra Groß, .; Christoph Lienau ,Quantum Matter,2014 ,3, 297-306
- Monajjemi, M. Chemical Physics. 2013, 425, 29-45
- Fazaeli ,R.; Monajjemi ,M.; Ataherian ,F.; Zare, K. Journal of Molecular Structure: THEOCHEM.2002, 581 (1), 51-58
- Monajjemi, M.; Mollaamin, F, J Clust Sci, 22(2011)673.
- Medhat Ibrahim and Hanan Elhaes ,Rev. Theor. Sci. 2013, 1, 368-376
- Anurag Srivastava.; Nileshi Saraf.; A. K. Nagawat , Quantum Matter,2013, 2, 401-407
- IIJIMA Sumio.; YUDASAKA Masako.; NIHEY Fumiyuki.; NEC TECHNICAL JOURNAL,2007, 2,1,
- S. Iijima and T. Ichihasi, Nature,1993,363, 603
- D. S. Bethune.;C. H. Kiang.; M. S. deVries.; G. Gorman, R. Savoy.; J. Vazques.; R. Beyers, Nature ,1993, 363, 605
- Monajjemi, M .; Sobhanmanesh, A .; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures,2013, 21 47–63
- Monajjemi ,M.; Karachi ,N.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, ,2014, 22: 643–662
- Monajjemi, M.; Mahdavian, L.; Mollaamin, F.: Bull .Chem.Soc.Ethiop ,2008, 22(2),1-10.
- V. Tozzini.; F. Buda.; A. Fasolino.; physical review letters ,2000,21,85
- G. Seifert.; P. W. Fowler.; Mitchell, D.; Porezag, D.; Frauenheim, Th. Chem. Phys. Lett. 1997, 268, 252
- Mollaamin ,F.; Baei, MT.; Monajjemi, M.; Zhiani , R.; Honarparvar , B.; Russian Journal of Physical Chemistry A, Focus on Chemistry,2008, 82 (13), 2354-2361
- Monajjemi, M.; Ghiasi, R. ;Ketabi, S. Journal of Chemical Research.2004, 1: 11-18
- Mahdavian,L.; Monajjemi, M.; Mangkorntong ,N.Fullerenes, Nanotubes and Carbon Nanostructures,2009, 17 (5), 484-495
- Mollaamin, F.; Gharibe, S.; Monajjemi, M. Int. J. Phy. Sci , 2011,6, 1496-1500
- Monajjemi, M .; Faham, R.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures , 2012 20, 163–169
- Monajjemi, M.; Khaleghian, M.; Tadayonpour, N.; Mollaamin, F. International Journal of Nanoscience, 2010, 9 (05), 517-529
- Mollaamin , F .; Najafi ,F.; Khaleghian, M.; Khalili Hadad, B.; Monajjemi ,M. Fullerenes, Nanotubes, and Carbon Nanostructures,2011 19, 653–667
- Monajjemi, M.; Chegini , H. ; Mollaamin , F. ; Farahani ,P, Fullerenes, Nanotubes, and Carbon Nanostructures.2011,19, 469–482
- Monajjemi, M.; Yamola ,H.; Mollaamin,F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 595–603
- Monajjemi, M.; Heshmata, M.; Haeri, HH , Biochemistry (Moscow),2006, 71 (1), S113-S122
- Monajjemi, M. Theor Chem Acc , 2015, 134:77 DOI 10.1007/s00214-015-1668-9
- A. Rubio.; J.L. Corkill.; M.L. Cohen.; Phys. Rev. B ,1994, 49, 5081
- X. Blasé.; A. Rubio.; S.G. Louie.; M.L. Cohen.; Europhys. Lett, 1994.28, 335.
- N.G. Chopra.; J. Luyken.; K. Cherry.; V.H. Crespi.; M.L. Cohen,S.G. Louie.; A. Zettl, Science.1995 , 269, 966
- N.G. Chopra.;A. Zettl.;Solid State Commun.1998 ,105, 297
- J. Cumings.; A. Zettl, Solid State Commun.2004, 129, 661
- R. Ma.; Y. Bando.; H. Zhu.; T. Sato, C. Xu, D. Wu, J. Am.Chem. Soc.2002, 124, 7672,
- P.W. Fowler, K.M. Rogers, G. Seifert, M. Terrones, and H. Terrones, Chem. Phys. Lett. 1999, 299, 359
- Monajjemi, M .; Falahati, M.; Mollaamin, F.; Ionics, 2013 , 19, 155–164
- Monajjemi , M.; Heshmat ,M.; Aghaei , H.; Ahmadi , R.; Zare,K. Bulletin of the Chemical Society of Ethiopia, 2007, 21 (1)
- Monajjemi , M.; Lee, V.S. ; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F, J. Phys.Chem. C. 2010, 114 (2010) 15315
- A. Rubio, J.L. Corkill, M.L. Cohen, Phys. Rev. B.1994, 49,5081
- X. Blase, A. Rubio, S.G. Louie, M.L. Cohen, Europhys. Lett. 1994, 28 335.
- N.G. Chopra, J. Luyken, K. Cherry, V.H. Crespi, M.L. Cohen,S.G. Louie, A. Zettl, Science ,1995, 269, 966.
- O.R. Lourie, C.R. Jones, B.M. Bartlett, P.C. Gibbons, R.S. Ruoff,W.E. Buhro, Chem. Mater.2000, 12 ,1808;
- R. Ma, Y. Bando, T.Sato, Chem. Phys. Lett.2001, 337 ,61.
- W. Han, Y. Bando.; K. Kurashima.; T. Sato, Appl. Phys. Lett.1998, 73, 3085;
- (a) D. Golberg.; Y. Bando.; M. Eremets.; K. Takemura.; K. Kurashima.;H.Yusa, Appl. Phys. Lett.1996 ,69, 2045
- D.P. Yu, X.S. Sun, C.S. Lee, I. Bello, S.T. Lee, H.D. Gu, K.M. Leung, G.W. Zhou, Z.F. Dong.; Z. Zhang.; Appl. Phys. Lett.1998, 72 , 1966.
- F.Jensen.; H.Toftlund, Chem. Phys. Lett.1993, 201,89 , 94
- Monajjemi, M .; Aghaie , H.; Naderi , F. Biochemistry (Moscow).2007, 72 (6), 652-657
- Monajjemi, M. Journal of Molecular Modeling , 2014, 20, 2507
- Davide Fiscaletti,Rev. Theor. Sci.2013 1, 103-144
- Monajjemi , M.; Chahkandi ,B.; Zare,K.; Amiri, A. Biochemistry (Moscow),2005 70 (3), 366-376
- Monajjemi, M .; Afsharnezhad ,S.; Jaafari , M.R.; Abdolahi ,T.; Nikosade ,A.; Monajemi ,H.; Russian Journal of physical chemistry A, 2007, 2,1956-1963
- Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22 ( 4 ), 673-692
- Mollaamin , F.; Monajjemi , M , Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
- Monajjemi, M. Struct. Chem, 2012, 23 551.
- Jon M. Matxain.; Jesus M. Ugalde.; M. D. Towler.; and R. J. Needs.; J. Phys. Chem. A 2003, 107, 10004-10010
- WU Haishun.;XU Xiaohong,; JIAO Haijun, Zhang Fuqiang .; JIA Jianfeng. Chinese Science Bulletin. 2003, 48, 11 1102 1107
- Monajjemi, M .; Ketabi ,S.; Amiri, A. Russian Journal of Physical Chemistry , 2006, 80 (1), S55-S62
- M. Monajjemi .; Robert Wayne Jr, J.E. Boggs, Chemical. Physics. 433 (2014) 1-11
- Jon M. Matxain.; Jesus M. Ugalde.; M. D. Towler.; and R. J. Needs.; J. Phys. Chem. A 2003, 107, 10004-10010
- WU Haishun.; XU iaohong.; JIAO Haijun, ZHANG Fuqiang .; JIA Jianfeng Chinese Science Bulletin 2003, 48 (11), 1102 1107
- Monajjemi , M.; Honarparvar, B.; Monajemi, H.;. Journal of the Mexican Chemical Society, 2006, 50 (4), 143-148
- Monajjemi ,M.; Mollaamin ,F. Journal of Computational and Theoretical Nanoscience,2012 ,9 (12) 2208-2214
- Monajjemi, M.; Mahdavian, L.; Mollaamin, F.; Honarparvar, B. Fullerenes, Nanotubes and Carbon Nanostructures, 2010, 18, 45–55
- Friedrich, B.; J.D. Weinstein.; R. Decarvalho .; J.M. Doyle.;. Trap. J. Chem. Phys. 1999, 110,2376-2383.
- Ramsay, N.E.; Magnetic Shilding of Nuclei. J. Phys. Rev.1950, 78, 699-703.
- Frischend, M.J.; J.B. Foresman, 1995. Gaussian 94 user’ reference (Gaussian, Inc., Pittsburgh).
- Ghalandari, B.; Monajjemi, M.; Mollaamin, F.; Journal of Computational and Theoretical Nanoscience, 2011 8, 1212–1219
- Monajjemi , M.; Khosravi , M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F. Physics and Chemistry of Liquids,2008, 46 299.
- Tahan, A .; Monajjemi, M. Acta Biotheor, 2011, 59, 291–312
- Monajjemi, M.; Farahani, N.; Mollaamin, F. Physics and Chemistry of Liquids, 2012, 50(2) 161–172
- Monajjemi, M.; Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.; Russian Journal of Physical Chemistry A , 2008 , 82 (13), 2277-2285
- Mollaamin , F.; Varmaghani , Z.; Monajjemi , M, Physics and Chemistry of Liquids. 2011, 49 318
- Monajjemi, M.; Honarparvar, B.; H. Haeri, H.; Heshmat, M.; Russian Journal of Physical Chemistry C, 2008, 80(1),S40-S44.
- Cheeseman, J.R.; M.J. Frisch.; F.J. Devlin .;P.J.Stephens, Chemical Physics Letters, 1996,252 (3-4), 211-220.
- Pisani, C.;S. Casassa .; P. Ugliengo, Chemical Physics Letter. 1996, 253 (3-4) 201-208.
- Dresselhaus, M.; Dresselhaus, G.; Eklund, P. C., Science of Fullerenes and Carbon Nanotubes, San Diego: Academic Press, 1996, 109, 175.
- Oku,T.; Nishiwaki ,A.; Narita,I.; Gonda,G.. Chemical Physics Letters, 2003, 380, 620–623.
- Nirmala,V.; Kolandaivel,P.; Journal of Molecular Structure, 2007, THEOCHEM, 817,137-145.
- J.C. Charlier, J.C.; Rignanese,G.M. Phys. Rev. Lett, 2001, 86, 5970.
- Naghsh,F, oriental journal of chemistry, 2015, 31(1) 465-478
- Chitsazan, A, oriental journal of chemistry, 2015, 31(1) 393- 408

This work is licensed under a Creative Commons Attribution 4.0 International License.









